BIOMET SpinalPak User manual
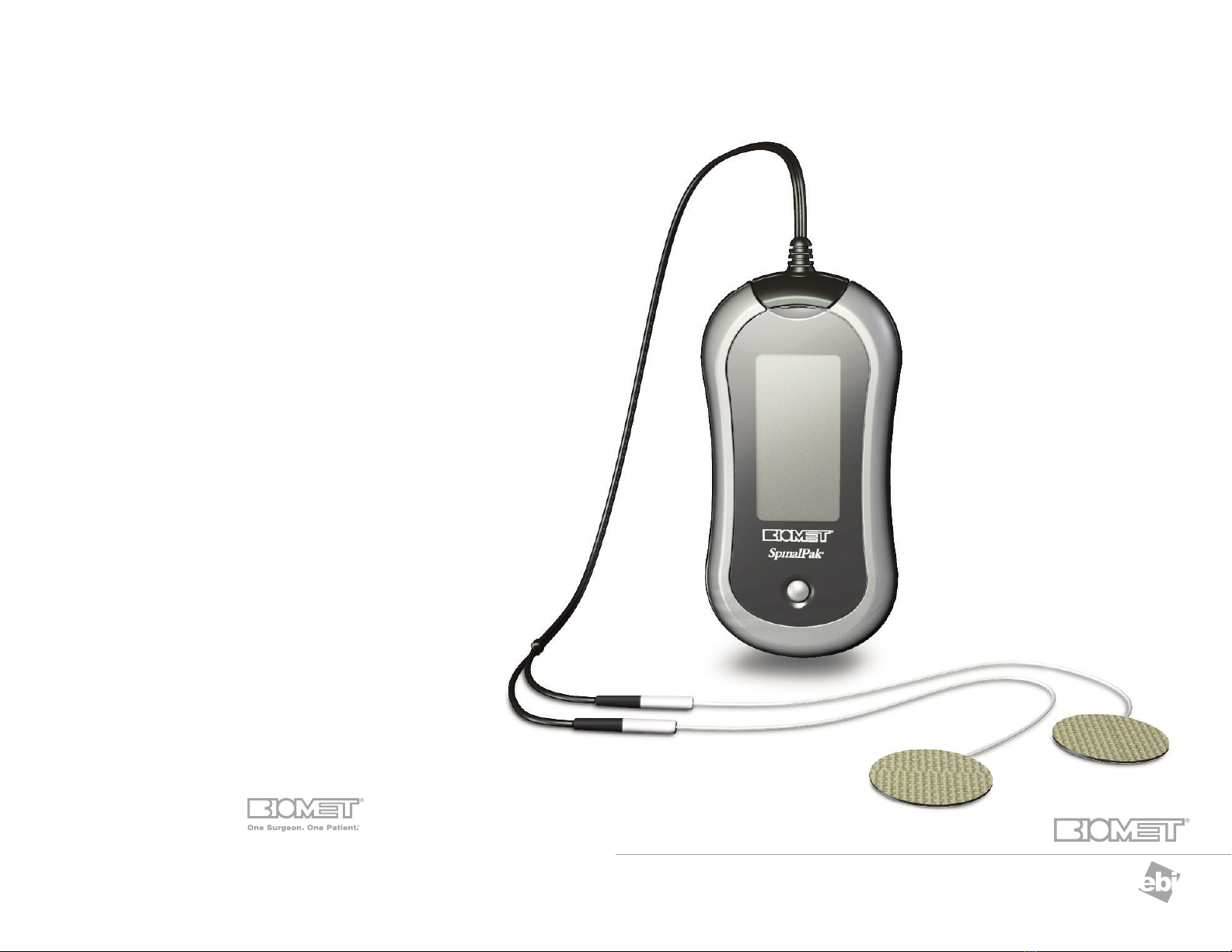
Biomet®SpinalPak®
Non- nvasive Spine Fusion Stimulator System
Complete Manual and Package Insert
™
Non- nvasive Stimulation
OPTIONS • EVIDENCE • EXPERIENCE
100 nterpace Parkway • Parsippany, NJ 07054
800-526-2579 • www.biomet.com • BNS231002L
©2009 EB , LLC. All trademarks are the property of Biomet, nc.
or its subsidiaries unless otherwise indicated. Rx Only.
1067795-00 REV C

1
able Of Contents
SYSTEM CONTENTS ..................................................................................................................1
MPORTANT SAFEGUARDS ........................................................................................................2
B OMET®SP NALPAK®ST MULATOR ........................................................................................3
• Description............................................................................................................................3
• Electrical Requirements for Battery and Charger ..................................................................3
SYSTEM COMPONENTS ..........................................................................................................3,4
FULL PRESCR B NG NFORMAT ON ..........................................................................................5
• ndications For Use ..............................................................................................................5
• Warnings, Precautions, Adverse Effects................................................................................5
D RECT ONS FOR USE................................................................................................................6
• Recommended Usage ..........................................................................................................6
OPERAT NG NSTRUCT ONS ......................................................................................................7
CHARG NG THE BATTERY PACK..............................................................................................7,8
BUTTON FUNCT ONS ..................................................................................................................9
LCD SYMBOL DESCR PT ON AND NSTRUCT ONS....................................................................9
TREATMENT COMPLET ON ......................................................................................................10
PAT ENT COMPL ANCE MON TOR NG ....................................................................................10
ORDER NG NFORMAT ON........................................................................................................10
SYMBOL DESCR PT ON............................................................................................................11
EQU PMENT CLASS F CAT ON..................................................................................................11
CLEAN NG NSTRUCT ONS ......................................................................................................11
ELECTROMAGNET C COMPAT B L TY ......................................................................12,13,14,15
PAT ENT COUNSEL NG NFORMAT ON ....................................................................................16
STORAGE & HANDL NG ..........................................................................................................16
D SPOSAL NSTRUCT ONS ......................................................................................................16
System Contents
• Electrodes - Soft Touch®- 72R (PN 106130-20)
• Electrodes - Soft Touch®- LT-4500 (PN 106130-12)
• Charger (PN 1067721)
• Batteries (2) (PN 1067720)
• Electrode Covers (PN 106130-17)
• Stimulator (PN 1067717)
• Device Holster (PN 1067722)
• Lead Wires - 12” Lead Wire (PN 1067724-01), 48” Lead Wire (PN 1067724-04)
• Patient Manual
Important Safeguards
READ ALL INSTRUCTIONS BEFORE USING
SAVE THESE INSTRUCTIONS
When using electrical products, basic safety precautions should always be followed, including:
A EN ION: o reduce the risk of electric shock, fire or injury:
1. Do not use this product while bathing, showering or swimming.
2. Do not place or store this product where it can fall or be pulled into a tub or sink.
3. Do not immerse the stimulator, battery charger or the battery in water.
4. Do not reach for this product if it has fallen into water. Unplug from the wall outlet immediately.
5. Do not permit the battery charger to be connected when wet.
6. Do not touch the battery contacts when the battery charger is plugged into an outlet.
7. Never operate the battery charger if it has a damaged power cord, plug or if it is not working
properly. Do not use if it has been dropped and damaged, or immersed into water. Contact
Biomet for return instructions.
8. Do not attempt to charge any other type of batteries in the Biomet®SpinalPak®Stimulator
battery charger.
9. Keep all cords away from heated surfaces
10. Never insert any foreign object into any opening of the system.
11. Do not expose the stimulator or the battery charger to prolonged heat or direct sunlight.
(Normal operating temperature range is 5°C to 38°C [41°F to 100°F], normal storage/transport
temperature is -15°C to 50°C [5°F to 122°F].
12. Use this product only for its intended use as described in this manual.
13. The Biomet®SpinalPak®Stimulator System has no installation, periodic maintainance
requirements or user serviceable parts. f any of the replacement parts are damaged they must
be replaced by Biomet in order to avoid a hazard.
14. Do not short circuit, overcharge, crush, mutilate, nail penetrate, heat, reverse the + or -
terminals or disassemble the battery. Do not allow metal objects to come into contact with the
battery terminals. These and any other abuses of the battery may cause serious injury and/or
burns. To ensure proper charging and safety, use only the charger supplied with your device.
Keep battery dry. This battery pack must be disposed of properly. Disposal information can
be obtained by contacting the Rechargeable Battery Recycling Corporation (RBRC) at
1-800-822-8837.
NO E: Inside the United States call Biomet at 1-800-526-2579 or 1-973-299-9300 if calling from
outside the United States with any questions or problems.
2
System Components
STIMULATOR
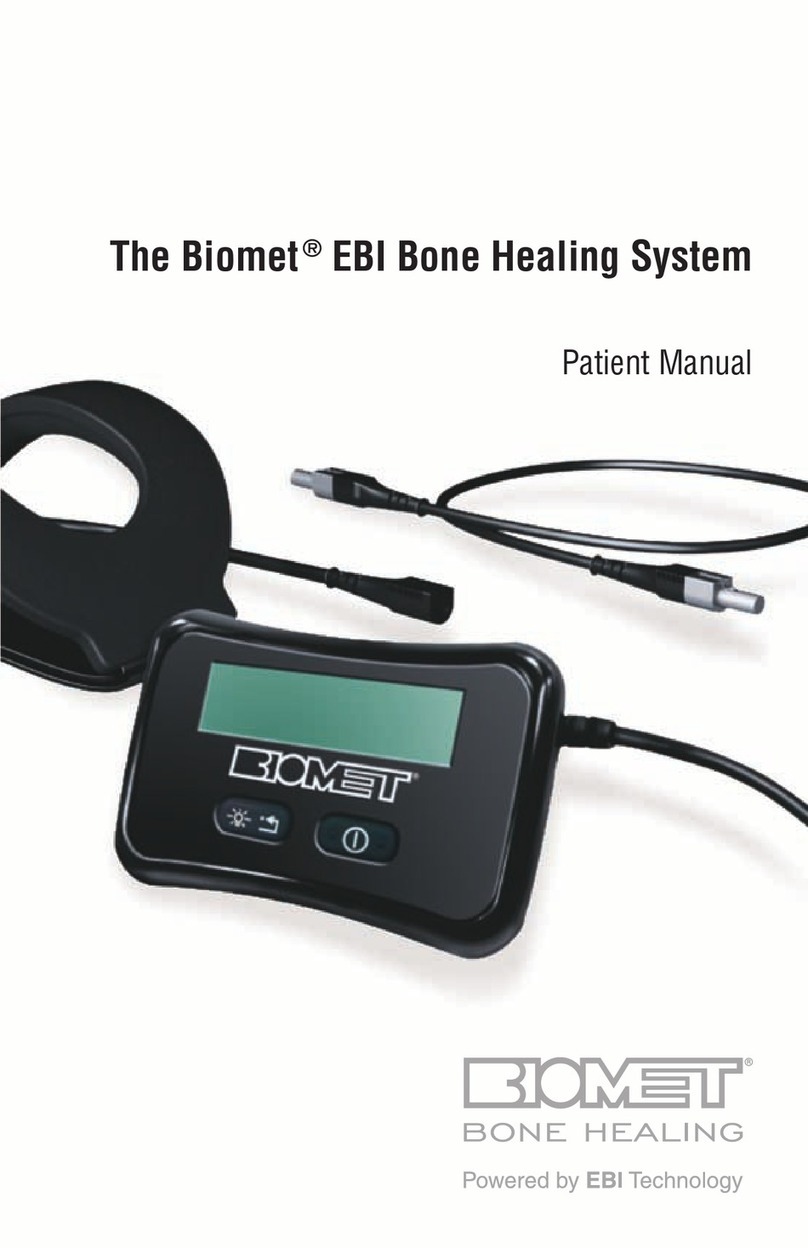
The Biomet®SpinalPak®Stimulator operates on a rechargeable battery, which allows for ambulatory use. t includes an
audible and visible self-checking alarm mechanism to alert the patient if it is not functioning properly. The Biomet®
SpinalPak®Stimulator is designed to store the patient’s daily therapeutic treatment data which may be downloaded and
read with the patient compliance software (See Patient Compliance Monitoring pg. 9). Patients are encouraged to bring
the stimulator to each follow-up visit with the prescribing physician to review how they are using their stimulator.
Biomet®SpinalPak®Stimulator
Caution: Federal Law (U.S.A.) restricts this device to sale by or on the order of a
physician. Rx Only.
This device is not intended for re-sale or re-distribution.
DESCRIPTION
The Biomet®SpinalPak®Stimulator (Figure 1) promotes healing by inducing a low electrical current
at the fusion site. The therapeutic signal generates a low energy electrical field by passing a specific
current between the electrodes.
ELECTRICAL RE UIREMENTS FOR BATTERY AND CHARGER
Charger
nput: 88-240 VAC 50/60 Hz 6 W
Output: 12V DC 500mA
For use with the Biomet®SpinalPak®Stimulator battery only (PN 1067720).
Battery rating: 3.7 VDC > 600 mAh
Do not use the batteries supplied with this unit in any other device.
Use of the Biomet®SpinalPak®batteries in any other device may cause damage or malfunction to the
batteries and/or device.
3
2
Figure 1

System Components (cont)
BATTERY PACK AND BATTERY CHARGER
The Biomet SpinalPak®Stimulator includes two rechargeable battery packs. Upon receipt, it is
recommended that the second battery pack be immediately placed into the charger and fully charged. n the mean-
time the first battery pack may be used to begin your treatment.
Note: The first battery pack may not pro ide a 24-hour treatment initially. It is recommended that the patient keep one
battery pack in the battery charger to assure that it is fully charged and ready, and the other inserted into the Biomet®
SpinalPak®Stimulator. This will ensure continuous treatment prescribed by the prescribing physician.
The battery charger is designed to recharge the Biomet®SpinalPak®Stimulator battery packs only. Two LED (light
emitting diode) lights monitor and indicate the operational status of the battery charger. The AC Power indicator
light is located on the AC Adaptor. The charging status indicator light is located on the charger
cradle. The following table lists and describes the operational functions of the LED lights:
Electrodes
There are two types of electrodes that are packaged with the Biomet®OrthoPak®Stimulator System
assembly: 72R and LT-4500. The 72R electrodes have green writing on their packaging. The LT-4500
electrodes have black writing on their packaging. The 72R electrodes have a hydrogel that is stickier
than the LT-4500 electrode hydrogel. The patient can use whichever electrodes best suit their skin type.
Electrode Covers
The electrode covers are water resistant and are intended to enhance electrode security to the skin,
if needed, or for showering with the electrodes attached, if desired.
Device Holster
The device holster is designed to securely hold the Biomet®SpinalPak®Stimulator in place. t has a
clip on the back which allows the patient to wear the device on their waistband or belt.
Lead Wires
Two different length lead wires are included with the Biomet®SpinalPak®Stimulator system. The
patient should choose the lead wire that best accommodates their needs for where they would like
to wear the control unit.
Following are possible error conditions and possible resolutions.
Error Conditions (flashes orange)
Battery pack not properly connected
to the charger
Battery temperature is too low or high
Battery voltage is too low
Possible Resolutions
Remove and re-install the battery pack to ensure
a complete connection to the charger
Normal operating temperature is
5˚C to 38˚C [41˚F - 100˚F]
Call Biomet for a new battery pack
Status AC Power
Indicator Light
Charging Status
Indicator Light
No battery pack inserted
(idle) on AC powered
battery charger
Solid green Off
Battery pack in charging state Solid green Solid orange
Fully charged battery pack Solid green Solid green
AC power deficiency Off Off
Error Solid green Off
4
Full Prescribing Information
INDICATIONS FOR USE
The Biomet®SpinalPak®Stimulator is a noninvasive bone growth stimulator indicated as an adjunct
electrical treatment to primary lumbar spinal fusion surgery for one or two levels.
WARNINGS
Cardiac pacemakers or cardioverters may be adversely affected by the Biomet®SpinalPak®Stimulator.
The concomitant use of the device and a pacemaker or cardioverter must be assessed on an
individual basis, such as with an electrocardiogram, prior to use. The patient should be referred to a
cardiologist for monitoring of pacemaker function while wearing the active Biomet®SpinalPak®
Stimulator device. f there are any observable adverse changes in the pacemaker rhythm or output, the
device should not be used.
The safety and effectiveness of the Biomet®SpinalPak®Stimulator in pregnant women have not been
studied, and the effects of the device on the mother or the developing fetus are unknown. A patient
who is either pregnant or is intending to become pregnant should be referred to her doctor prior to
treatment with the device.
PRECAUTIONS
The safety and effectiveness of the Biomet®SpinalPak®Stimulator in individuals with the following
conditions have not been studied, and therefore the safety and effectiveness of the device in these
individuals are unknown:
– spondylitis, infection, Paget’s disease
– cancer, diabetes mellitus, renal disease
– trauma of the lumbar spine
– osteoporosis.
Apply the electrodes after the skin has been cleaned and dried. f erythema develops at the
electrode sites, the electrodes should be relocated adjacent to the original sites. f the reaction does
not resolve after 48 hours after relocating the electrodes, the patient should be instructed to consult
with the physician.
Do not submerge or expose the Biomet®SpinalPak®Stimulator to water. The patient must be
instructed to remove the stimulator during bathing, showering or swimming.
Compliance with the treatment schedule, daily battery pack changes, and replacing the electrodes
(1 to 7 days) as needed are essential for proper device function. This system should only be used with
components and replacement parts supplied by Biomet. Other components, parts and accessories
may not be compatible, and may damage the device. f any component does not function properly,
contact Biomet. No attempt should be made to modify or repair the device.
Patients should be able to use the device in accordance with the instructions for use. If a patient
cannot comply with these instructions for any reason, use of the device is not recommended.
ADVERSE EVENTS
During a multi-center clinical study of 349 patients treated with the device for the indication listed
above, skin irritation was the most common adverse effect associated with the use of the device. t
occurred in 9 patients (2.6% of the trial population): 4 patients treated with the active device and 5
patients treated with the placebo device.
5
Directions For Use
RECOMMENDED USAGE
The Biomet®SpinalPak®Stimulator is designed to deliver 270 days of continuous therapeutic treat-
ment for 24 hours per day. The recommended daily therapeutic treatment is continuous for 24 hours.
OPERATING INSTRUCTIONS
The Biomet®SpinalPak®Stimulator has been specifically designed to be convenient to use, comfort-
able to wear, and safe to operate. Patients should begin using the Biomet®SpinalPak®Stimulator
immediately after reading the instructions for use and having received instructions from their
prescribing physician.
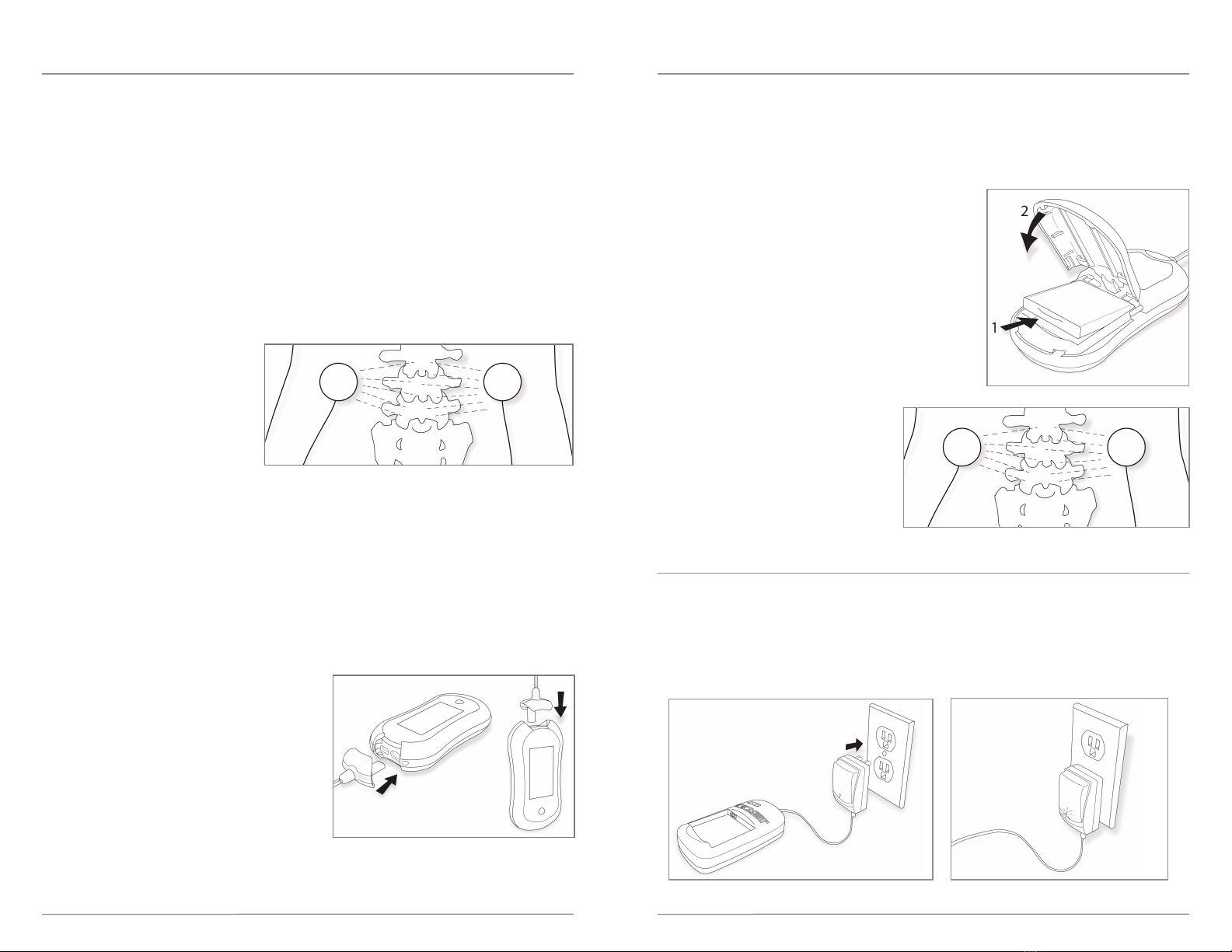
To begin treatment - Electrode Placement
• Clean and dry the skin where the electrodes will be placed. Trimming (not shaving) body hair from
the electrode application area is often helpful.
•Note: To ensure electrode placement and adhesion, you may use one of the pro ided electrode
retainer patches. Place one electrode
two to three inches to the left of the
area of the surgery and a second
electrode two to three inches to the
right of the area of the surgery so that
the electrodes are four to six inches
apart. See Figure 2. Depending on the
patient’s ability to move after surgery,
it may be helpful for the patient to ask
another person to assist them in placing these electrodes. See instructions for use on the electrode
packet. The patient should consult their prescribing physician or Biomet if they have any questions
or concerns regarding proper electrode placement. f their skin becomes abnormally red at the
electrode sites, the electrodes should be moved adjacent to the original sites. f the redness does
not go away after 48 hours with the electrodes removed, the patient should contact their
prescribing physician.
Tips:
Loose electrodes – Confirm that both electrodes are in complete contact with clean, dry skin. See
instructions on the electrode packet.
Incomplete circuit/disconnection – Check all connection points, confirming a snug fit.
Broken electrode lead wire – f alarming
continues after confirming connection, attach a new
electrode lead wire.
To begin treatment - Lead Wires
• The patient is provided with two stimulator lead wire
lengths with the Biomet®SpinalPak®Spine Fusion
Stimulator system. The patient should select the
length of the stimulator lead wire in order to
provide both convenience and comfort when
wearing the stimulator during treatment.
• nsert the stimulator lead wire male connection into each female electrode lead wire connection.
• nsert the lead wire plug into the opening at the top of the stimulator. See Figure 3.
Figure 2
Figure 3
6
Operating Instructions
Charging the Battery Pack
Charge the battery pack at room temperature (24˚ C (75˚ F)). Charging may require two to three hours.
Charging may vary in warmer or colder temperatures.
STEP 1:
Plug the battery pack charger into a wall outlet (Figure 5). A green light on the AC adaptor will
illuminate indicating power (Figure 6).
STEP 1:
nsert the charged battery pack into the Biomet®SpinalPak®
Stimulator (Figure 4). The LED light on top of the device will
blink, indicating power. Each symbol will be indicated on the
display and the alarm will flash and beep if the electrodes are
not properly applied. To silence the audio alarm press the
button below the display.
f the light does not blink, which indicates that the battery is
not charged, change the battery pack (See Charging the
Battery Pack below).
STEP 2:
Attach electrodes as per instructions on the
package, and as per “To Begin Treatment -
Electrode Placement” section on page 6.
7
Both battery packs provided with the Biomet®SpinalPak®Stimulator are charged prior to being
packaged and distributed. Upon receipt of the Biomet®SpinalPak®Stimulator, it is recommended that
the second battery pack be immediately placed into the charger and completely charged. n the
meantime, the first battery pack may be used to begin your treatment immediately. Note: The first
battery pack may not pro ide a complete 24-hour treatment initially.
Figure 4
Figure 5 Figure 6
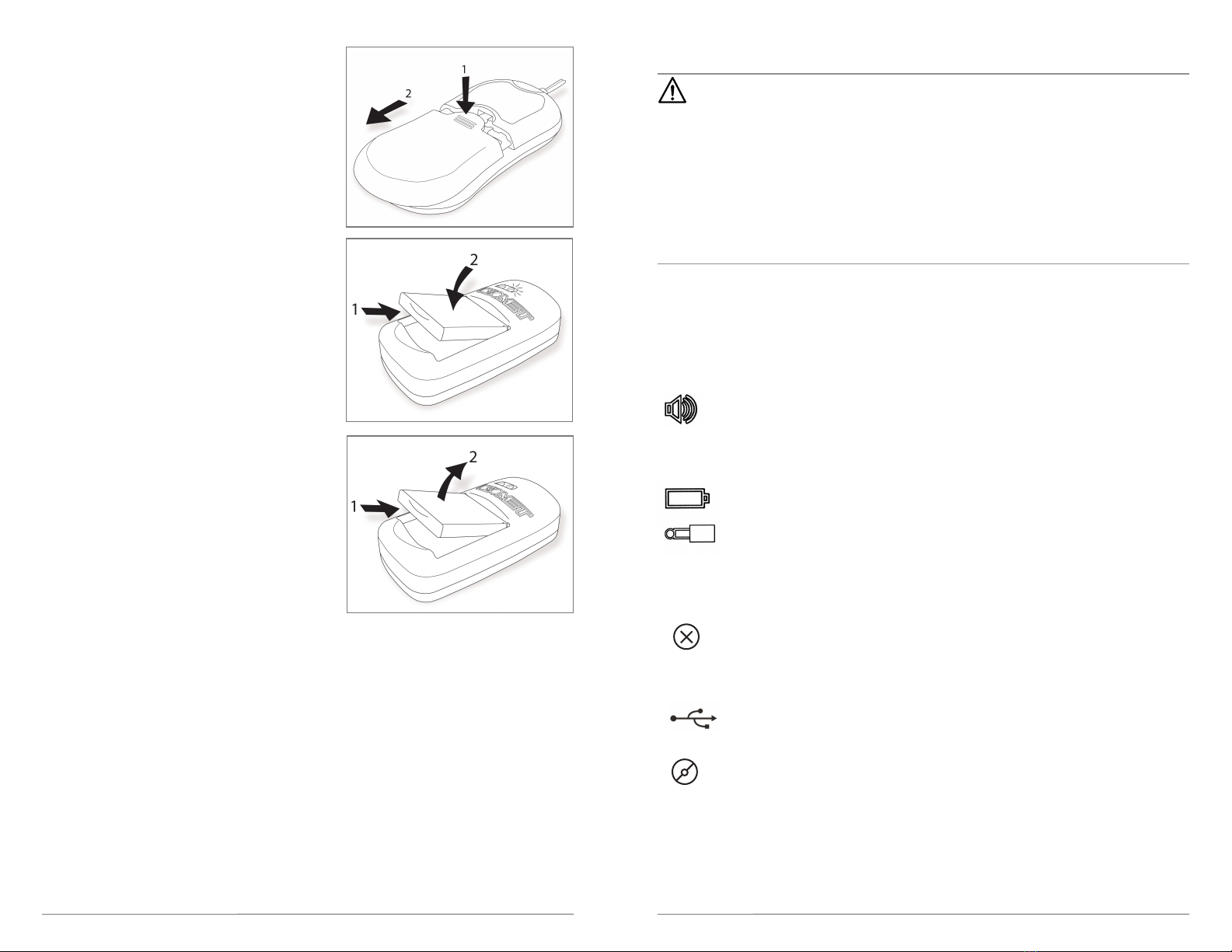
STEP 2: CHANGING BATTERY PACKS
Each day, preferably at the same time to ensure
treatment is continued without interruption, patients
should do the following:
A. Slide down the battery door on the back of the
stimulator and remove the depleted battery
(Figure 7).
B. Place the depleted battery pack into the battery
charger for charging (Figure 8). A solid orange light
on the charger will illuminate, indicating the battery
is charging. f no light appears on the charging
cradle an error is indicated. f this occurs, try
removing the battery pack from the charger and
reinserting it. f the orange light does not appear
contact Biomet.
C. Once the charger’s orange light turns off and
a solid green light appears, the battery pack is fully
charged. Remove the battery pack from the battery
charger (Figure 9) and place the fully charged battery
pack into the Biomet®SpinalPak®Stimulator in order
to commence treatment.
D. There should always be one battery in the charger
and one battery installed in the stimulator at all times,
ensuring a fully charged battery every 24 hours as
recommended.
NO E: Do not be concerned if the battery packs are inadvertently charged more than once or kept
on the charger for a long period of time. he batteries cannot be overcharged. If the battery pack
is in the battery pack charger and the battery pack is fully charged, the charger will terminate the
recharging process. he charger having no orange light when the charging is complete will
indicate this termination. Additional replacement battery packs are available by contacting
Biomet.
8
Figure 7
Figure 8
Figure 9
Button Functions
ALARM ON/OFF BUTTON
The Biomet®SpinalPak®Stimulator is activated as soon as a charged battery is inserted. The button
located below the LCD display enables or disables the audible alarm. During an alarm condition,
depressing the button quickly (0.5 seconds) will temporarily disable the audible alarm. Depressing the
button for a longer period of time (3 seconds) will toggle the audible alarm between enabled and dis-
abled. Patients should be advised to leave the audible alarm enabled as frequently as possible in order
to assure the fully prescribed treatment. A speaker symbol will be indicated on the LCD display when
the alarm is enabled.
The alarm defaults to audible alarm. Press the button below the display on the front of the stimulator
to silence the alarm. After silenced, the light will continue to flash and the display will indicate the
alarm condition.
Symbol/TEXT Condition Instructions
Treating Continue use.
Audible alarm f beeping, depress the button
briefly to silence the alarm. Depress the
button approximately 3 seconds to
engage or disengage the audible alarm.
Low battery nsert a charged battery pack.
Disconnection Confirm that each electrode is properly
applied on the skin. See the electrode
pouch for instructions. Confirm that the
lead wire is attached properly. Replace
the lead wire if necessary.
System error Error in the stimulator –
Contact Biomet for assistance.
Stimulator is Stimulator will not treat until USB
connected to cable is disconnected
a PC
End of operation Contact Biomet
LCD Symbol Descriptions and Instructions
9

reatment Completion
Therapeutic treatment should not be suspended until fusion occurs or until
such time as a determination is made by the prescribing physician that no
progression to fusion is occurring. The device is programmed to deliver 270
continuous days of therapeutic treatment and automatically discontinues
operation after the 270 days.
Patient Compliance Monitoring
The Biomet®SpinalPak®Stimulator contains embedded software which allows the display of patient
specific history data including usage and therapeutic treatment times. This data may be downloaded
to a personal computer for viewing, storage and/or print out via the use of Biomet Compliance Data
Download Software. Please call your local Biomet representative to obtain more information.
10
Ordering Information
To order supplies, contact Biomet. See page 2 “ mportant Safeguards” for contact information.
The following information is necessary to expedite any inquiry:
• Patient name
• Physician name
• Address to send replacement parts (patient home, MD office, etc.)
Attention see instructions
Alternating Current
Direct Current
Type B
Storage/Transport
temperature limits
Class
Non Sterile
Manufacturer
WEEE
Single Patient Use/
Prescription Only
Prescription Only
Warning: The concomitant
use of the stimulator and a
pacemaker or cardioverter
must be assessed by a
cardiologist on an
individual basis with an
Electrocardiogram (EKG).
Caution: The safety of
this device used during
pregnancy and nursing in
humans has not been
established.
Symbol Description
Equipment Classification
• Stimulator - nternally powered by rechargeable batteries
• Charger - Class , Type B
• Ordinary Equipment without protection against ingress of water
• Equipment not suitable for use in presence of flammable anesthetic mixture with air or oxygen
or nitrous oxide.
• Mode of operation - continuous
Cleaning Instructions
Use a damp cloth for cleaning any part of the Biomet®SpinalPak®Stimulator System. Do not use
cleaning products or detergents.
11
2
NON
STERILE
Rx only
RF emissions
C SPR 11
Electromagnetic Compatibility
A. The use of accessories, cables or replacement parts other than those supplied by Biomet
may result in increased emissions or decreased immunity of the equipment or system.
B. This equipment should not be used adjacent to or stacked upon other equipment.
C. Portable and mobile RF communications equipment can adversely affect the operation of
Medical Electrical Equipment.
D. n the event this equipment interferes with the operation of other equipment, or
experiences interference from other equipment, to continue treatment, it will be necessary
to move the Biomet®SpinalPak®Stimulator away from the source of the
interference as indicated in Table 4.
Guidance and manufacturer's declaration -
electromagnetic emissions
The Biomet®SpinalPak®Stimulator is intended for use in the electromagnetic
environment specified below. The customer or the user of the Biomet®SpinalPak®Stimulator
should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
Group 1
The Biomet®SpinalPak®Stimulator uses RF
energy only for its internal function. Therefore, its RF
emissions are very low and not likely to cause any
interference in nearby electronic equipment.
RF emissions
C SPR 11
Class B
The Biomet®SpinalPak®Stimulator is suitable
for use in all establishments, including domestic
establishments and those directly connected to the
public low-voltage power supply network that supplies
buildings used for domestic purposes.
Harmonic
emissions
EC 61000-3-2
Not applicable
Voltage
fluctuations/
flicker emissions
EC 61000-3-3
Not applicable
Table 1
12
Guidance and manufacturers declaration - electromagnetic immunity
The Biomet®SpinalPak®Stimulator is intended for use in the electromagnetic
environment specified below. The customer or the user of the Biomet®SpinalPak®
Stimulator should assure that it is used in such an environment.
mmunity test EC 60601
test level
Electromagnetic environment -
guidance
Electrostatic
discharge (ESD)
EC 610004-2
± 6 kV contact
± 8 kV air
Floors should be wood, concrete or
ceramic tile. f floors are covered with
synthetic material, the relative humidity
should be at least 30 %.
Electrical fast
transient/burst
EC 61000-4-4
Not Applicable
Surge
EC 61000-4-5 Not applicable
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
EC 61000-4-11
Not applicable
Power frequency
( 50/60 Hz )
magnetic field
EC 61000-4-8
Not applicable
Compliance level
Group 1
Class B
Not applicable
Not applicable
Not applicable
Table 2
13
Guidance and manufacturers declaration - electromagnetic immunity
The Biomet®SpinalPak®Stimulator is intended for use in the electromagnetic
environment specified below. The customer or the user of the Biomet®SpinalPak®
Stimulator should assure that it is used in such an environment.
mmunity test EC 60601
test level
Electromagnetic environment -
guidance
Conducted RF
EC 61000-4-6 Not Applicable
Radiated RF
EC 61000-4-3
3 V/m
80 MHz to 2.5
GHz
Portable and mobile RF communications equipment
should be used no closer to any part of the Biomet®
SpinalPak®Stimulator, including cables, than the recom-
mended separation distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance
d = 3.5 √ P 80 MHz to 800 MHz
d = 7 √ P 800 MHz to 2.5 GHz
where P is the maximum power output rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey,a should be
less than the compliance level in each frequency range.b
nterference may occur in the vicinity of equipment
marked with the following symbol:
NOTE 1. At 80 MHz and 800 MHz, the higher frequency applies.
NOTE 2. These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects, and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones
and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be
predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF
transmitters, an electromagnetic site survey should be considered. f the measured field strength in
the location in which the Biomet®SpinalPak®Stimulator is used exceeds the applicable RF compliance
level, the Biomet®SpinalPak®Stimulator device should be observed to verify normal operation. f
abnormal performance is observed, additional measures may be necessary, such as reorienting or
relocating the Biomet®SpinalPak®Stimulator.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 1 V/m.
Compliance level
Not Applicable
1 V/m
Table 3
14
Recommended separation distances between portable
and mobile RF communications equipment and the
Biomet®SpinalPak®Spine Fusion Stimulator
The Biomet®SpinalPak®Stimulator is intended for use in an electromagnetic environment in which
radiated RF disturbances are controlled. The customer or the user of the Biomet®SpinalPak®
Stimulator can help prevent electromagnetic interference by maintaining a minimum distance
between portable and mobile communications equipment (transmitters) and the Biomet®SpinalPak®
Stimulator as recommended below, according to the maximum power output of the
communications equipment.
Rated maximum
output power of
transmitter
W
Separation distance (meters)
according to frequency of transmitter
150 kHz to 80
MHz
d = 3.5 √ P
80 MHz to 800
MHz
d = 3.5 √ P
800 MHz to 2.5
GHz
d=7√P
0.01 .35 .35 .7
0.1 1.1 1.1 2.21
1 3.5 3.5 7
10 11.06 11.06 22.13
100 35 35 70
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer.
NOTE 1. At 80 MHz and 800 MHz, the separation distance for the higher frequency applies.
NOTE 2. These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects, and people.
Table 4
15

Patient Counseling Information
The patient should be thoroughly instructed on how to properly use and care for the Biomet®
SpinalPak®Stimulator and receive the patient’s guide, which provides detailed instructions.
A summary of the key points in the patient labeling is provided below.
Compliance - The patient should be instructed that compliance with device use and care is critical
to assure the proper function of the device and effective treatment.
Battery - The patient should be instructed to insert a fully charged battery into the stimulator
every 24 hours.
Electrodes - The patient should be instructed to replace the electrodes when needed, and to clean
the electrode sites thoroughly with soap and water prior to applying the electrodes.
Skin Irritation - The patient should be instructed to examine the skin for irritation when replacing
the electrodes. f irritation is present, the patient should be instructed to relocate the
electrodes adjacent to the original sites. The patient should be evaluated periodically
to assess the skin for sensitivity.
Alarms - See LCD Symbol Descriptions and nstructions (page 9). The patient should
be instructed to keep the audible alarm system engaged as often as practical, and to
engage the alarm system if it has been disengaged as soon as practical.
Bathing - The patient should be instructed to disconnect the stimulator during bathing,
showering or swimming. t should be reconnected as soon as practical following
these activities. The patient should also be instructed to either remove the
electrodes, or to cover the electrodes with the protective retainer patches, during
showering.
Storage and Handling
The Biomet®SpinalPak®Stimulator should be stored in a cool and dry place. The device components
should be handled with care. Damage may occur if the device is inappropriately handled or abused.
Disposal Instructions
When treatment has concluded as determined by the prescribing physician (see page 10), Biomet
requests that the patient disposes of the Biomet®SpinalPak®Stimulator according to local statutes
and regulations.
16 17
Notes:
Other manuals for SpinalPak
1
Table of contents
Other BIOMET Medical Equipment manuals
Popular Medical Equipment manuals by other brands

DemeTECH
DemeTECH DemeMASK N95 quick start guide

PFM Medical
PFM Medical pfm Stretching Table 1100 operating manual

Enabling Technologies
Enabling Technologies Dynamique Biski owner's manual

Promeba
Promeba PA-535 instruction manual
ResMed
ResMed AirFit N20 user guide
Gaumard
Gaumard Susie Simon S200 user guide