PAGE 2
COPYRIGHT © 2020 AES TECHNOLOGIES
Introduction
Foreword & Motivation
The AES Technologies COVID-19 PCV Ventilator device was designed specifically to help address the dire
ventilator shortage due to the COVID-19 Pandemic. As of this writing, on April 1, 2020, New York City is
the current epicenter of this virus, and over 900 deaths were reported in the US within a 24-hour period.
This indicates that the death rate in the US is currently doubling every three days - on par to quickly
outpace even Italy, who was hit extremely hard a couple of weeks prior to the US.
It appears that the aside from the obvious calamity and complete lack of preparedness for such a
widespread catastrophe, the primary mechanisms of death in the COVID-19 Pandemic will likely be due
to:
•ARDS
•Lack of respiratory support (ventilators, respiratory therapists) to combat the influx of ARDS
patients to the ICU
oLack of PPE contributing to dwindling personnel
The shortage of ventilators is a massive dilemma that boils down to a lack of parts/components, and an
inability of the supply chain to address the shortage quickly enough - especially for New York.
Our device was designed to address these shortages. There is a massive supply and supply chain for
CPAP/BiPAP machines prescribed for Sleep Apnea. These machines in their stock form do not have the
capability of functioning as PCV Ventilators (aside from hospital-grade units), but they do have the
necessary electromechanical components to function as such - and are already FDA approved medical
devices. Our conversion kit utilizes the electromechanical components of the existing CPAP/BiPAP
machines, and adds in a closed-loop pressure regulating control system, combined with a therapist-
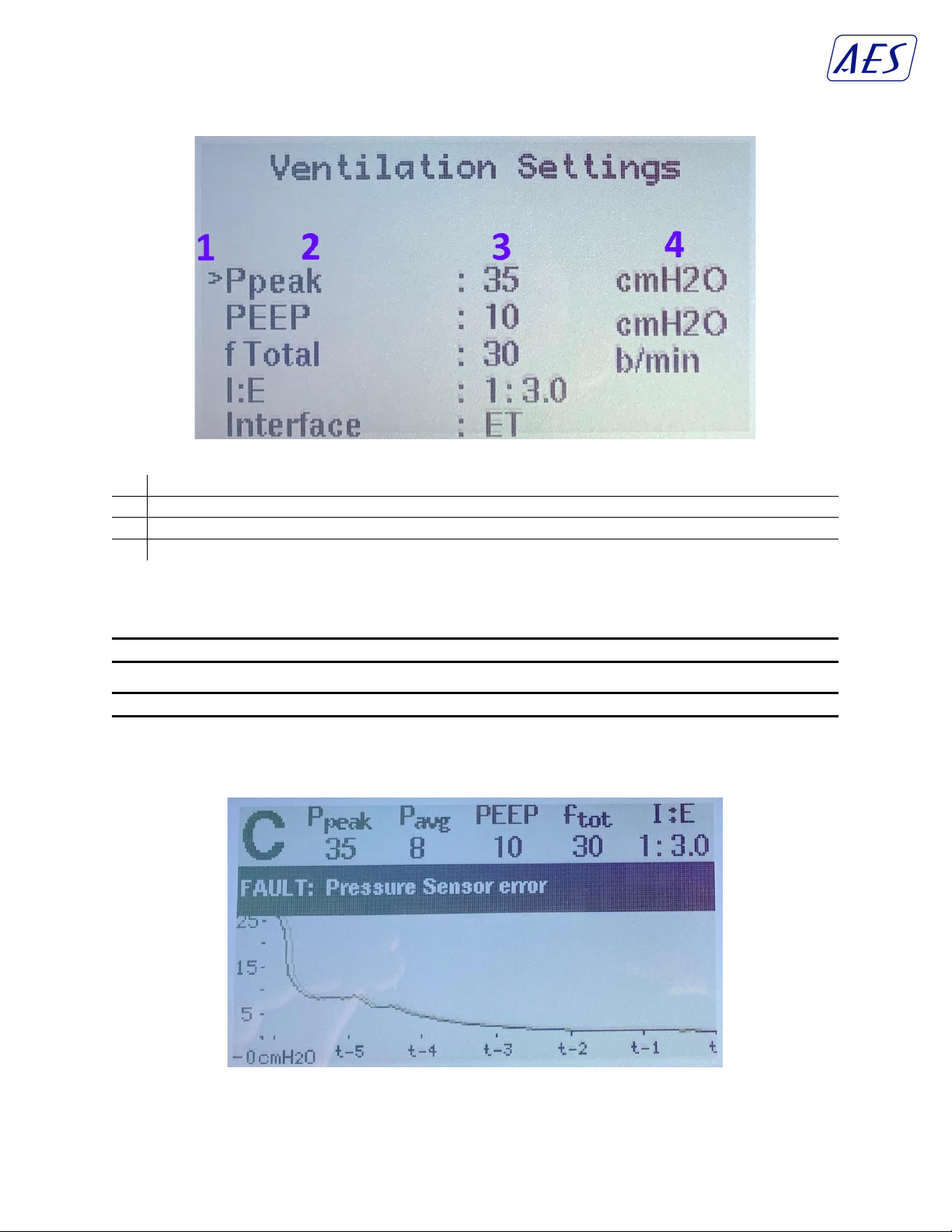
programmable interface to control ventilator mode (control or support mode), peak pressure, PEEP,
breathing rate (b/min), and Inspiratory:Expiratory ratio (I:E). This converts the device into a PCV
Ventilator capable of supporting up to 35 cmH2O of pressure, and a programmable PEEP with a high
settable limit - with the understanding that ARDS patients need substantial PEEP to maintain alveoli
recruitment and to prevent pneumothorax.
Another chief concern with such a conversion - especially in the case of non-invasive ventilation (NIV) - is
the spread of aerosol or droplets from the open-loop circuits present in these CPAP/BiPAP machines.
We have designed an anti-aerosolization system that utilizes existing standard parts, including
exhalation ports and inline antibacterial/antiviral filters. This ensures that minimal droplets/aerosol
escape the closed-loop system, even in NIV cases (of course, we recommend the added precaution of a
surgical mask over the NIV mask in any case).
Lastly, this design was established to address this need in HIGH VOLUME. Using a small Bill of Materials
(BOM) of very easily obtainable parts (and parts that do not overlap with the existing invasive ventilator
(IV) supply chain), virtually any existing supply of CPAP/BiPAP machines can be converted for immediate
use as a PCV Ventilator.