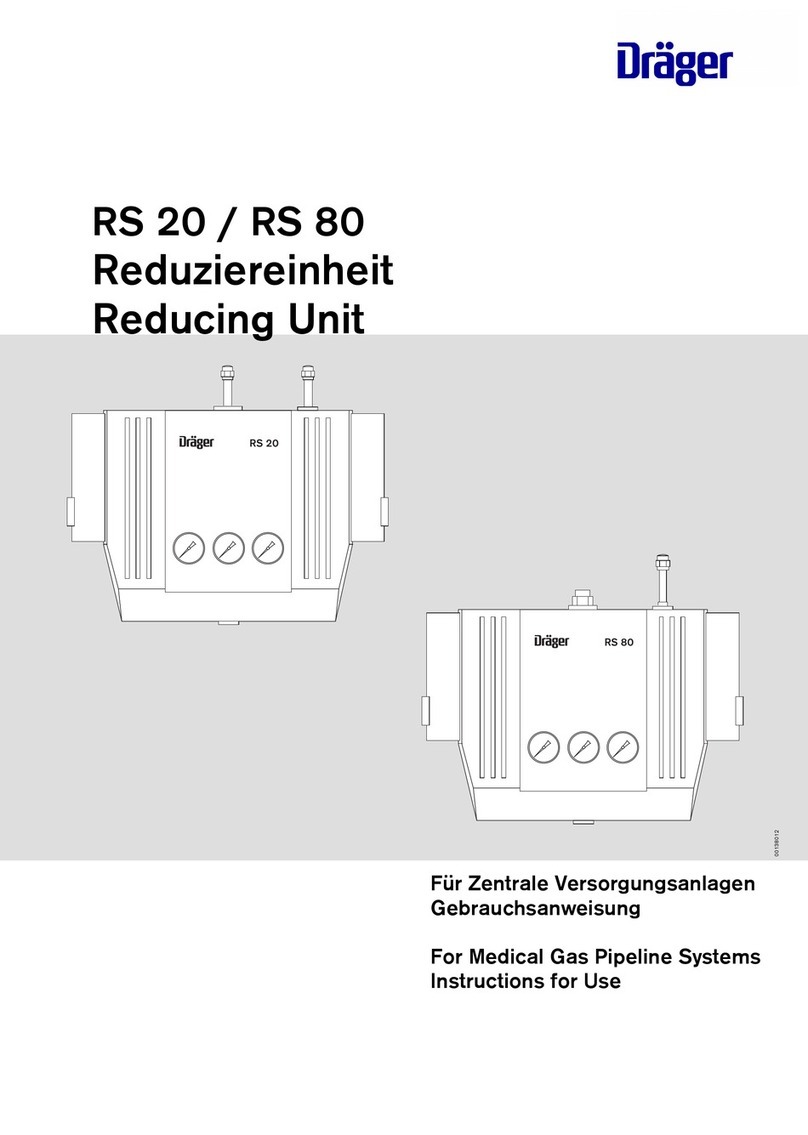
Bien-Air Dental MC2 User manual

MC2 & Isolite hoses
Do not sterilize
ENG INSTRUCTIONS FOR USE.
Other languages available on
https://dental.bienair.com/IFU
Rx Only
REF 2100017-0002/2022.10

REF 2100017-0002/2022.10 HOSE MC2 - Isolite • © Bien-Air Dental SA
Devices (REF)
HOSE ISOLITE/MC2 GREY
REF 1600120-001
HOSE ISOLITE/MC2 COIL ED BL ACK
REF 1600315-001
HOSE ISOLITE SWIVEL GREY
REF 1600298-001
HOSE ISOLITE WATER ADJ GREY
REF 1600134-001
HOSE ISOLITE SWIVEL GREY
REF 1600132-001
Optional accessories
O-RING 2.5x1.5
REF 705.02.08-010
MAINT SPRAYNET® (BOX OF 6 CANS)
REF 1600036-006

1 Symbols 4
1.1 Description of symbols used 4
2 Identification & Intended Use 5
2.1 Identification 5
2.2 Intended use 5
2.3 Intended patientpopulation 5
2.4 Intended user 5
2.5 Use environment 5
2.6 Intended medicalconditions 5
2.7 Patient contra-indications
and side effects 5
2.8 In case of accident 5
3User and Patient Safety:
Warnings and Precautions
for use 6
4 Description 8
4.1 Overview 8
4.2 Assembly and preparation 9
4.3 Assembly 11
4.4 Technical data 13
4.5 Classification 13
4.6 Performances 13
4.7 Operating conditions 13
5 Maintenance and servicing 14
5.1 Maintenance – General in-
formation 14
5.2 Cleaning 14
5.3 Rinsing 14
5.4 Drying 14
5.5 Packing and storage 14
5.6 Servicing 15
6 Transport & disposal 15
6.1 Transport 15
6.2 Disposal 15
7 General information 16
7.1 Terms of guarantee 16
8 References 16
3
Table of contents

4
ENG INSTRUCTIONS FOR USE
1 Symbols
1.1 Description of symbols used
Symbol Description Symbol Description
Manufacturer. Catalogue number.
CE Marking wit h number of the notified body. Consult instructions for use or consult electronic
inst ruc tions for use .
W ARNING: haz ard tha t c ould result in s erious injury
or damage to the device if the safety instructions
are not correctly followed.
Medical Device.
CAU TION : hazard that could result in light or
moderate injury or damage to the dev ic e if the
safety instructions are not correctly followed.
Authoriz ed EC Representative in the European
Community.
Wear protective gloves . Batch c ode.
Data Matrix code for product inf ormation
including UDI (U nique Device Identification). Temperature limit.
Humidity limitation. Atmospheric pressure limitation.
Keep away from rain. Ge neral sy mbol for recov ery/re cy cla ble.
W arning: in ac cordanc e with f ederal
law ( USA) , this dev ic e is only available for s ale upon
recommendation by an accredited practitioner.
Recyclable electrical and electronic material.

ENG
2 Identification &
Intended Use
2.1 Identification
Medical devices manufactured by Bien-
Air Dental SA.
Type:
HOSE ISOLITE/MC2 GREY
Grey hose with fixed connector.
HOSE ISOLITE/MC2 COILED BLACK
Black coiled hose with fixed connector.
HOSE ISOLITE/MC2 SWIVEL GREY
Grey hose with revolving connector.
HOSE ISOLITE WATER ADJ GREY
Grey hose with ring to adjust water
spray flow.
HOSE ISOLITE SWIVEL GREY
Grey swivel hose with revolving con-
nector.
Those hoses are intended to be used
with motors ISOLITE LK & ISOLITE LED
(from this point on referred to as MC2
motor type).
Description:
Hoses are essential accessories meant
to connect motors to the con-
soles/electrical drive motor board.
2.2 Intended use
Product intended for use in general
dentistry which includes restorative
dentistry, dental prophylaxis and or-
thodontics treatments.
2.3 Intended patient
population
The intended patient population for the
device includes any person visiting a
dental practitioners’ office to receive
treatment in line with the intended med-
ical condition. There is no restriction
concerning subject age, race, or cul-
ture. The intended user is responsible
to select the adequate device for the pa-
tient according to the specific clinical
application.
2.4 Intended user
Product intended for professional use
only. Used by dentists and dental pro-
fessionals.
2.5 Use environment
Professional healthcare facility en-
vironment.
2.6 Intended medical
conditions
General dentistry which includes res-
torative dentistry, dental prophylaxis,
orthodontics and addresses the main-
tenance or reestablishment of dental
health.
2.7 Patient contra
-indications and side effects
No specific patient contra- indication,
side effects nor warning exist for the
device when it is used as intended.
2.8 In case of accident
If an accident occurs, the device must
not be used.
If any serious incident occurs in relation
to the device, report it to a competent
authority of your country, as well as the
manufacturer through your regional
distributor. Observe relevant national
regulations for detailed procedures.
WARNING
Any use other than that for which this
device is intended is unauthorised and
may be dangerous.
5

3 User and Patient
Safety: Warnings and
Precautions for use
This medical device must be used by
professionals in compliance with the
legal provisions in force regarding oc-
cupational safety, health and accident
prevention measures, and these in-
structions for use.
In accordance with these provisions, the
user is responsible for ensuring he or
she only uses devices which are in per-
fect working order.
Electrical safety:
WARNING
Electrical safety in conformance with
IEC 60601-1 can only be claimed when
the device is used with Bien-Air Dental
compatible devices (drive motors and
motors). In addition, only medical
power supply with 2 MOPP should be
used.
Electromagnetic compatibility:
WARNING
lElectromagnetic compatibility can
only be claimed when the device is
used with Bien-Air Dental com-
patible devices (drive motors and
motors).
lSince compliance with the in-
ternational standard IEC60601-1-
2 does not guarantee immunity
against 5G worldwide (due to the
different frequency bands used
locally), avoid the presence of
devices equipped with 5G broad-
band cellular networks in the
clinical environment or ensure
that the network functionality of
these devices is disabled during
the clinical procedure.
To prevent any risk of explosion, the
warnings below must be observed:
WARNING
According to IEC 60601- 1:2005 +A1
2012 / AnnexG, electrified devices (mo-
tors, control units, couplers and
attachments), can be safely used in a
medical environment in which po-
tentially explosive or flammable
mixtures of anaesthetic substances are
delivered to the patient only if:
lThe distance between the motor
and the anaesthetic breathing cir-
cuit exceeds 25 cm.
lThe motor is not used sim-
ultaneously to the administration
of the anaesthetic substances to
the patient.
To prevent any risk of infection, the
warning below must be observed:
WARNING
lMedical personnel using or per-
forming maintenance on medical
devices that are contaminated or
potentially contaminated must
comply with universal pre-
cautions, in particular the wearing
of personal protective equipment
(gloves, goggles, etc.). Pointed and
sharp instruments should be
handled with great care.
6

ENG
To prevent any material damage the
cautions below must be observed:
CAUTION
lDo not use the hose to pull the
unit or the cart. This misuse could
damage the internal wires and/or
the external sheath.
lIt is essential to use dry, purified
compressed air in the dental unit
in order to ensure the long work-
ing life of the device. Maintain the
quality of the air and the water by
regular maintenance of the com-
pressor and the filtration systems.
The use of unfiltered hard water
will lead to early blockage of the
tubes and connectors.
7

4 Description
4.1 Overview
FIG.1
(1) Sheath
(2) Reinforced part of the sheath
(3) Motor connector
Note : The technical specifications, illustrations and dimensions contained in these
instructions are given merely as an indication. They may not give rise to any claim.
The original language of those instructions for use is English.
For any further information, please contact Bien-Air Dental SA at the address
given on the back cover.
8
FIG.1

ENG
4.2 Assembly and preparation
Pictogram used
Move in the direction indicated. Move f ully to the s top, in the
direction indicated.
1. Check that the rear of the motor and the hose connector are clean and dry.
2. Connect (without the split ring) the motor to the proprietary hose as shown in
FIG.2.
3. Rotate it to find the exact position and push it into the motor.
4. Holding the motor fully screw the hose sleeve to the rear motor connection
(FIG.3).
9
FIG.2 FIG.3

ENG
4.3 Assembly
FIG.5
Preserve the initial alignment of the wires and tubes. Insert the hose in the Dental
unit fixation placing the reinforced part of the hose (10) in the chucking zone
(FIG.5a).
The securing cord (9) must be attached to the chassis of the unit or table-top
device to avoid any traction on the wires and tubes (FIG.5b).
The outer tube must not be wrinkled after installation. The resistance to traction is
60N maximum.
Description FIG.5
1. Ø 1.5/2.5 mm air spray (small white) with “A” or (blue)
2. Ø 1.5/2.5 mm water spray (small white) with “W” or (green)
3. Ø 1.5/2.5 mm motor cooling (small white) or (transparent) Ø2.8/4.1 mm
4.4. (+) red: motor
5.5. Black: motor
6.6. (+) brown: bulb
7.7. (0V) blue: bulb
8.8. Back-up ring
9. Securing cord
10. Metal reinforcement of the sheath
11
FIG.5

ENG
4.4 Technical data
CAUTION
These hoses are not suitable for pressure higher than 5 bar (500 kPa, 72 psi).
Standard length
1.7 or 2 m
Special length
3 m
Note : See the technical data of the micromotor MOT ISOLITE LED (REF 1600681-
001) or of the micromotor MOT ISOLITE LK (1600078-001) for more information.
4.5 Classification
Class IIa in accordance with the European Medical Regulation (EU) 2017/745.
4.6 Performances
No performances related to the hose alone. Refer to the IFU of the compatible mi-
cromotor MOT ISOLITE LED (REF 1600681-001) or MOT ISOLITE LK (1600078-001).
4.7 Operating conditions
Operating conditions
Temperature range: +10° C — +35° C (+50° F — +95° F)
Re lat iv e hum idity range : 30% — 80%
Air pressure range: 700 hPa — 1060 hPa
13

14
5 Maintenance and servicing
5.1 Maintenance – General information
CAUTION
lNon-sterilizable.
lNever submerge the hose in disinfectant solutions (the connectors should
never be completely submerged).
lDo not use an ultrasonic cleaner.
5.2 Cleaning
Clean with a clean cloth moistened with either tap water, sterile demineralized (de-
ionized) water or any appropriate product for dissolving protein and blood
residues.
5.3 Rinsing
Remove the product residues with a clean cloth soaked either in tap water or in
sterile demineralized (deionized) water.
5.4 Drying
Spray the exterior of the hose with Spraynet® then remove its excess with a non-
woven cloth. Do not use products containing acetone, chlorine or bleach.
5.5 Packing and storage
Storage conditions
Temperature range: 0° C — +40°C (+32° F — +104° F)
Re lat iv e hum idity range : 10% — 80%
Air pressure range: 650 hPa — 1060 hPa
Keep away from rain
The device must be stored in a dry and dust free environment.
CAUTION
If the medical device has been stored refrigerated, allow it to warm up to room
temperature prior to its use.

ENG
5.6 Servicing
Bien-Air Dental SA recommends that the user change the hose every two years.
CAUTION
Never disassemble the device. For any inquiry, contact your regular supplier or
Bien-Air Dental service centre.
6 Transport & disposal
6.1 Transport
Transport conditions
Temperature range: -20°C — +50° C (-4°F — +122°F)
Re lat iv e hum idity range : 5% — 80%
Air pressure range: 650 hPa — 1060 hPa
Keep away from rain
6.2 Disposal
The disposal of this device must be performed in accordance with the legislation in
force.
This device must be recycled. Electrical and electronic equipment may contain dan-
gerous substances which constitute health and environmental hazards. The user
must return the device to its dealer or establish direct contact with an approved
body for treatment and recovery of this type of equipment (European Directive
2012/19/ EU).
15

16
7 General information
7.1 Terms of guarantee
Bien-Air Dental SA grants the operator a guarantee covering all functional defects,
material or production faults.
The warranty period is:
l12 months from the date of invoicing.
In the event of justified claim, Bien-Air Dental or its authorised representative will
fulfil the company’s obligations under this guarantee by repairing or replacing the
product free of charge.
Any other claims of any kind whatsoever, particularly claims for damage or injury
and the consequences thereof resulting from:
lExcessive wear and tear
lInfrequent or improper use
lFailure to observe the servicing, assembly or maintenance instructions
lDamage caused by unusual chemical, electrical or electrolytic influences
lFaulty air, water or electrical connections
Are excluded.
CAUTION
The warranty becomes null and void if damage and its consequences result from in-
correct servicing or modification by third parties not authorized by Bien-Air Dental
SA. Warranty requests will only be taken into consideration if the product is ac-
companied by a copy of the invoice or delivery note. The following information must
be clearly indicated: purchase date, product reference and serial number.
8 References
REF Legend
1600120-001 HOSE ISOLITE/MC2 GREY
1600315-001 HOSE ISOLITE/MC2 COILED BLACK
1600298-001 HOSE ISOLITE/MC2 SWIVEL GREY
1600134-001 HOSE ISOLITE WATER ADJ GREY
1600132-001 HOSE ISOLITE SWIVEL GREY
705.02.08-010 O-RING 2.5x1.5
1600036-006 Spraynet®, cleaning spray 500 ml, box of 6 cans

Bien-Air Dental SA
Länggasse 60 Case postale 2500 Bienne 6 Switzerland
Tel. +41 (0)32 344 64 64 Fax +41 (0)32 344 64 91
dental@bienair.com
Other addresses available at
www.bienair.com
Bien-Air France Sàrl
19-21 rue du 8 mai 1945
94110 Arcueil
France
REF 2100017-0002/2022.10 HOSE MC2 - Isolite • © Bien-Air Dental SA
Table of contents
Other Bien-Air Dental Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Stryker
Stryker Adel 2100 Childbearing Bed Operation manual

ElproSys
ElproSys DiagProg4 instruction manual

Nasco Healthcare
Nasco Healthcare Simulaids Life/form Baby Buddy instruction manual

Storz
Storz ENDOMAT SELECT UP 210 instruction manual

Spencer
Spencer STX 518 Use and maintenance manual

Dräger
Dräger X-plore 1900 NIOSH manual