Biobeat BB-613WP User manual

Monitoring Wristwatch | Disposable Patch
User Guide

Important
This User Manual is subject to periodic review, update and
revision.
Do not use a defective product. Do not repair this product or any
of its parts other than in accordance with written inructions
provided by Biobeat.
The user of this product has sole responsibility for any
malfunction that results from improper use, faulty maintenance,
improper repair, unauthorized service, damage, or alteration by
anyone other than Biobeat Technologies Ltd.
The safety, reliability, and performance of this device can only
be assured under the following conditions:
• The device has been used according to the accompanying
operating instructions.
• All fittings, extensions, readjustments, changes, or repairs
have been carried out by Biobeat Technologies Ltd.
authorized representatives.
No part of this publication may be reproduced, ored in a
retrieval syem or transmitted in any form by any means,
electronic, mechanical, photo reproductive, recording or
otherwise without the express prior written permission of
Biobeat Technologies Ltd.
Biobeat Technologies Ltd. reserves the right to change or
improve its products and accompanying technical literature
without specic notice of changes or improvements.
This product is protected by the following US patent
applications:
US20180020960(A1) PCT/IL2017/050752
and other pending US patents.
Caution: Consult with a physician before use of the device.
Disclaimer
Information provided by Biobeat Technologies Ltd. is believed
to be accurate and reliable. However, Biobeat Technologies Ltd.
assumes no responsibility for the use of such information, nor
for any infringements of patents or other rights of third parties,
that may result from its use.
Ce symbol CE indicates compliance of this - device with the In
Vitro Diagnoic Medical Device Directive 98/79/EC.
belis s.a.
Bd General Wahis 53
1030 Brussels, Belgium
Tel: +(32) 2 732-59-54
Fax: +(32) 2 732-60-03

1. ABOUT THIS USER MANUAL
This User Manual provides the information necessary to operate
the Biobeat Syem.
PLEASE READ THIS USER MANUAL BEFORE OPERATING
THE SYSTEM. If any part of this User Manual is not clear,
contact Cuomer Support for assiance.
1.1. TYPES OF WARNINGS, CAUTIONS AND NOTES
Three types of special messages appear in this User Manual:
¡Warning: A warning indicates precautions to avoid the
possibility of personal injury or death.
Caution: A caution indicates a condition that may lead
to damage to equipment, or a lower quality of treatment.
ǂNote: A note provides other important information.
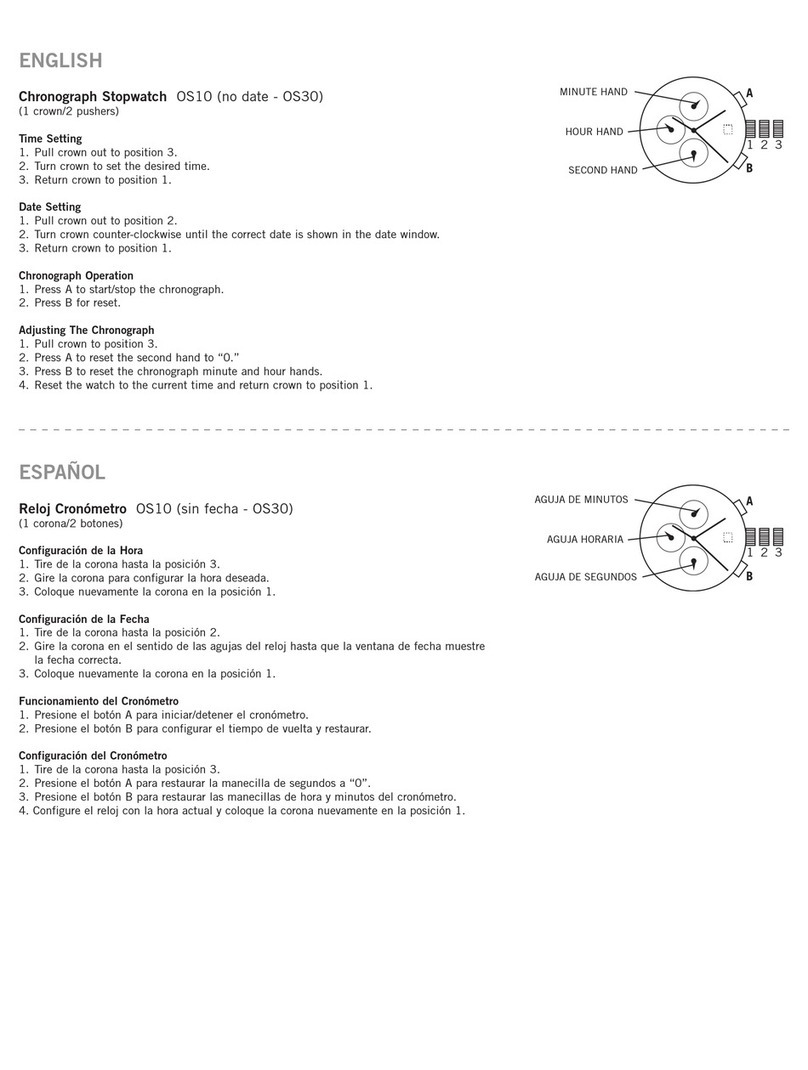
2. OVERVIEW OF SYSTEM
2.1. Description of Device
The BB-613WP is a non-invasive wriwatch or patch used for
monitoring of vital signs in clinical and non-clinical settings.
Features and benets:
• Easy of use.
• Ability to transmit the data to the App.
• BLE Communication.
3. CONDITIONS FOR USE
3.1. Indications
A baseline reference measurement of blood pressure and heart
rate should be entered into the Web Platform before use of the
device. The baseline reference measurement is performed using
a andard blood pressure cu-based oscillometric device.
Note: Standard blood pressure is considered to be an average
of 3 consecutive measurements.
The BB-613WP is intended for spot-checking of adult patients
in hospitals, clinics, long-term care, and home use.
3.2. Contraindications
¡Do not use with neonatal or pediatric patients.
¡If the BB-613WP is mechanically damaged it must not be
used and must be disposed.
¡Do not use with patients with significant deformity,
swelling, irritation, degenerative changes or edema of the
wrist.

¡Do not use with patients with localized infection, ulceration
or skin lesions involving the wrist.
¡Do not use with patients that have restricted blood flow
e.g. tourniquet, pressure cuff or IV line.
¡Do not use with patients with tremors or convulsions.
¡Do not use with patients with peripheral vascular disease
affecting the hands.
¡Do not use on an area with a tattoo.
¡DO NOT use in MRI or a CT environment.
¡Do not use if there is a known allergy to metals, plastic
and silicon.
4. SAFETY
4.1. Electrical Safety
The device complies with the requirements of AAMI/ANSI/IEC/
EN 60601-1+ED-3 for safety of medical equipment:
Class II equipment type BF applied part.
Mode of operation: spot measurement.
Degree of mobility: portable.
4.2. EMC Compliance
The device complies with the requirements of IEC/EN 60601-1-
2+ED-4 for EMC of medical equipment:
The device has Class BF III compliance.
4.3. Safety Instructions
Warnings:
¡DO NOT USE BEFORE READING THIS USER MANUAL.
¡Only apply the device on clean, intact wrist skin.
¡The device can only measure while the patient is at rest.
¡This device is not defibrillation proof per IEC60601-1.
¡Do not use the device in an MR environment or in an
explosive atmosphere, such as in the presence of a
flammable anesthetic.
¡In case of discomfort, inspect the device sensor application
site to ensure correct sensor alignment and skin integrity.
¡Avoid excessive pressure to the sensor application site as
this may cause damage to the skin beneath the sensor
(device straps too tight).
¡Regarding the frequency of sensor relocation and
inspection of application site: The device must be located
on the wrist to activate the device properly. Check the
application site every 4 hours for skin integrity. If there is
any concern, remove the device and replace with another

device on the other hand.
¡Do not use the device if you have known allergy to plastic,
silicon and metals.
¡If a skin reaction appears following the use or during the
use of the device, stop using the device immediately.
¡This device is intended only as an adjunct in patient
assessment.
¡The system contains no user-serviceable components.
¡Do not immerse the device in water or any other liquid.
¡Diseases with peripheral circulatory disturbance may cause
incorrect readings.
¡The pulse rate indicator is not suitable for monitoring the
frequency of cardiac pacemakers.
¡The device must be able to measure the pulse properly to
obtain an accurate SPO2 measurement. Verify that nothing
is hindering the pulse measurement before relying on the
SPO2 measurement.
¡General operation of the device may be affected by the
use of an electrosurgical unit (ESU). This device should not
be used adjacent to other equipment. If adjacent use is
necessary, the device should be observed carefully to verify
normal operation.
¡The device is intended for indoor operation.
¡The device should not be used as a substitute for a
laboratory blood analyzer.
¡Excessive pressure from the device for prolonged periods
can induce pressure injury.
¡Do not use the device outside the declared environmental
conditions (see Section 12. Specifications). Operating the
device outside the declared environmental conditions can
lead to incorrect measurements.
Cautions:
The watch is for adult patients with wrist a circumference
between 18-25 cm.
Disposal of this device should be performed in accordance
with local regulations.
If the temperature or humidity is outside of the
recommended range (see Section 12. Specifications), do
not use the device.
If the display on the device is not working properly, or the
On/Off button is faulty, as shown in Section 5.2. Detailed
Description of Watch (Front), do not use the device.
Table of contents
Popular Watch manuals by other brands

Casio
Casio QW 5513 Operation guide

Piaget
Piaget 560P Instructions for use

Armitron
Armitron pro sport MD0346 instruction manual

West Marine
West Marine BlackTip 13411293 Instruction Booklet and Care Guide

Jaeger-leCoultre
Jaeger-leCoultre HYBRIS MECHANICA CALIBRE 184 manual

FOREVER
FOREVER iGO PRO JW-200 user manual