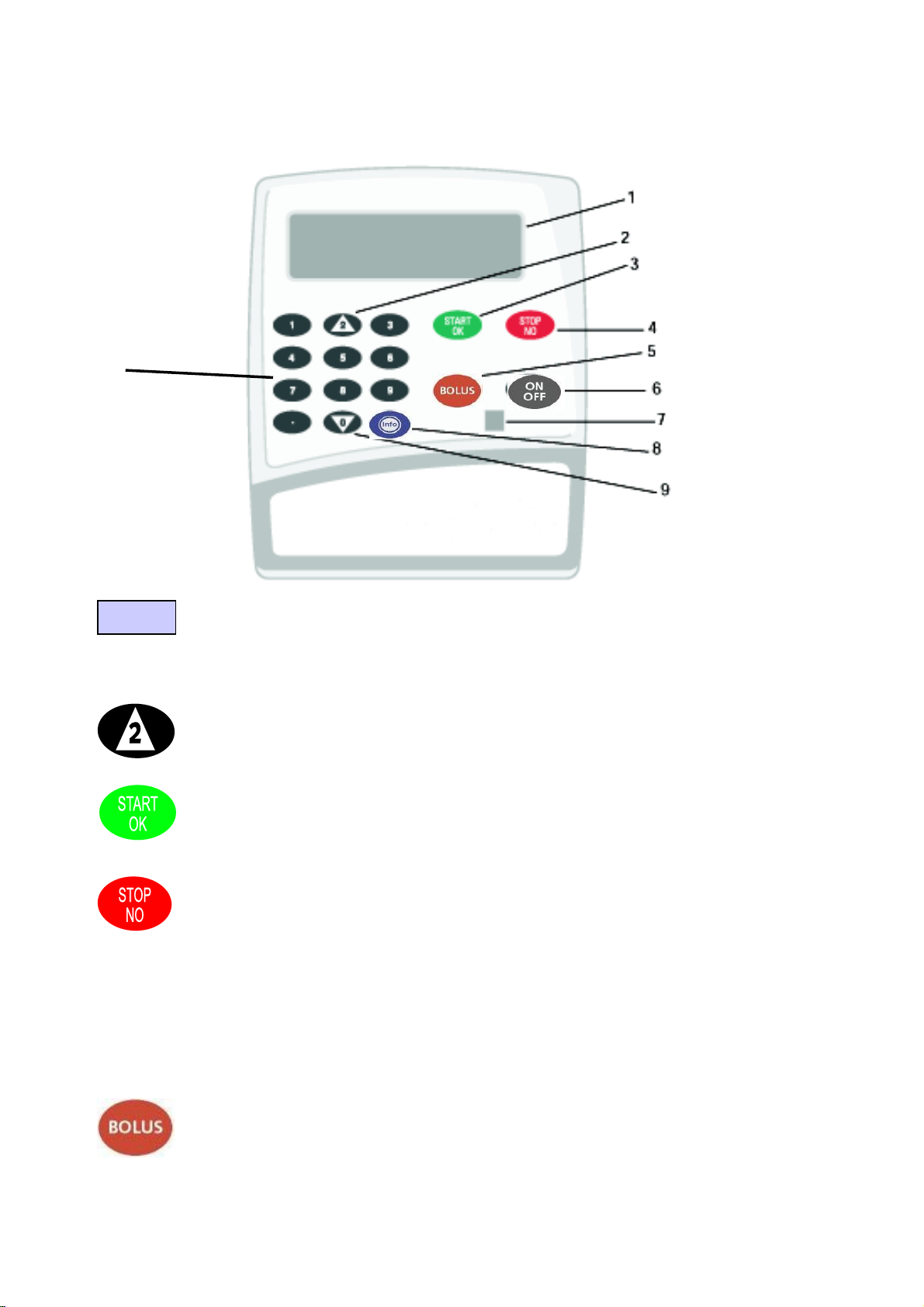
CME Medical BodyGuard™ 545 Epidural Infusion Pump Operaon Manual/Rev 2.3/October 2013
10
OPERATING PRECAUTIONS
Although the BodyGuard pump has been designed and manufactured to exact specicaons, it is not
intended to replace trained personnel in the supervision of epidural infusions.
CME Medical will assume no responsibility for incidents which may occur if the product is not used,
stored or transported in accordance with the environmental condions spulated in this document
and on the package labelling.
This infusion pump is designed for ambulatory use, and should withstand everyday handling. If the
pump is dropped onto a hard surface, or is suspected of being dropped, the operaon and calibraon
should be checked by a qualied technician.
Do not bathe or shower whilst using the pump or immerse into liquid. The pump is resistant to a lim-
ited amount of splashing, but its construcon does not make it resistant to large amounts of spraying
or immersion in liquids. Damage to the internal components may result.
The BodyGuard pump should be operated within a temperature range of +18ºC (+59ºF) to +45ºC
(+113ºF) and up to 85% humidity. Operang the pump at temperatures and/or humidity other than
within that range may aect accuracy.
Do not operate the pump near high-energy radio-frequency eming equipment, such as electro-
surgical cauterising equipment. False alarm signals may occur.
INFUSION PRECAUTIONS
Always read and follow the instrucons which accompany the administraon sets. Carefully follow
the instrucons for priming the set, as well as the recommended set change interval.
The uid bag and administraon set should be disposed of in an appropriate manner, considering the
nature of the residual uid that may be contained within, in accordance with the hospital/homecare
provider’s disposal pracces.
Drugs for infusion by use of the pump may only be prescribed by a qualied medical praconer.
Cauon must be exercised in the selecon of drugs and the amount and rate intended to be delivered
via any infusion pump.
If the drug contained in the uid bag will be exposed to extreme environmental condions for pro-
longed me periods, it is important to select drugs that will not change pharmacologically upon such
exposure.
As with all automac infusion devices, whenever a toxic or dangerous level of drug is stored in the
reservoir, constant monitoring of the infusion is required.
In all applicaons, me to alarm under occlusion or other fault condions will depend on the infusion
rate and levels of alarm sengs. It is recommended to consider these parameters when using drugs
requiring infusion stability or low ow rates, and therefore a quick me to alarm.
Disposables must be compable with the medicine delivered.
When operang the pump on paent controlled programme with a rate of 0.00ml/h there is a hazard
of blood clot forming. Connecng a clear uid infusion in parallel will avoid this problem.
GENERAL PRECAUTIONS
Do not use hard or sharp objects on the keypad.
The specied accuracy of the pump can only be maintained if the pump is used, maintained and ser-
viced in accordance with the instrucons given in this manual. If the pump has failed to calibrate dur-
ing the servicing procedure, it must be returned for repair or disposal.
If the pump is dropped, subjected to excessive moisture, humidity or high temperature, or otherwise
suspected to have been damaged, remove it from service for inspecon by qualied personnel.
The pump has been designed to be as safe as possible to handle; however, care should be exercised
to avoid trapping of ngers or other body parts in the mechanism.