ELITechGroup MACRODUCT ADVANCED 3710 SYS User manual

MACRODUCT®
ADVANCED
MODEL 3710 SYS
USER’S MANUAL
Sweat Collection System


MACRODUCT® ADVANCED
SWEAT COLLECTION
SYSTEM
Model 3710 SYS
User’s Manual
57-0192-01E (Last update 17 June 2021)

2
©2018 ELITechGroup Inc. All rights reserved.
Printed in the United States of America. No part of this publication may be reproduced,
transmitted, transcribed, stored in a retrieval system, or translated into any language (human or
computer) in any form, or by any means whatsoever, without the prior express written permission
of ELITechGroup Inc.
Webster Sweat Inducer™, Sweat-Chek™, and EasyDuct™ are trademarks of ELITechGroup Inc.
Wescor®, ChloroChek®, Chloridometer®, Pilogel® and Macroduct® are registered trademarks.
Other trade names used in this manual are trademarks of their respective owners, used here for
information only.
ELITechGroup Inc. makes no express or implied warranty regarding this manual, its quality,
performance, or appropriate use regarding any type of specific procedure. ELITechGroup Inc. may
modify this manual without notice and without implying any obligation or liability on the part of
the company.
Manufactured in the United States of America by:
ELITechGroup Inc.
370 West 1700 South
Logan, Utah 84321-8212 USA
The Notified Body number 2797 above signifies that British Standards Institute BSI has certified
the Production Quality Assurance System of ELITechGroup Inc., according to Annex V of the
Medical Device Directive 93/42/EEC (MDD). The scope of that certificate, CE 59518, is:
The manufacture of sweat analysis systems for cystic fibrosis; and sweat inducers (to obtain
samples for use in the subsequent laboratory diagnosis of cystic fibrosis).
This covers the Class IIa devices Macroduct Advanced Model 3710, Macroduct Model 3700, and
Nanoduct Model 1030. Together with the Declaration of Conformity issued by the manufacturer
according to Annex VII, this allows the CE marking of these devices. There are no accessories to
which the CE certificate or the BSI Notified Body Number 2797 applies.
Macroduct Advanced Sweat Collector patent number:
US9226730 B2
Also published as:
EP2973536A2, US20140276220, WO2014145904A2, WO2014145904A3
2797

3
Table of Contents
Section 1
Introduction
1.1 Device Overview ............................................................................................................ 5
Using this Manual................................................................................................. 5
Specific Warnings ................................................................................................. 6
Contraindications ................................................................................................. 7
Functional Description ......................................................................................... 8
Key Features......................................................................................................... 9
Intended Use........................................................................................................ 9
Table 1: Explanation of Symbols........................................................................... 10
1.2 Device Description ......................................................................................................... 12
Figure 1: System Components .............................................................................. 12
Figure 2: Display................................................................................................... 13
Touchscreen ......................................................................................................... 13
Display.................................................................................................................. 13
Figure 3: Top Panel............................................................................................... 14
Figure 4: Model/Serial Number Identification Label ............................................ 15
Figure 5: Electrode Cable Assembly...................................................................... 15
Figure 6: Pilogel Discs........................................................................................... 15
Figure 7: Macroduct Advanced Sweat Collector................................................... 16
Figure 8: Small Sealable Containers ..................................................................... 16
Figure 9: Collector and Electrode Straps............................................................... 16
Figure 10: Battery Charging Power Supply and AC Power Cord
for Battery Charging............................................................................................ 17
Figure 11: EasyDuct Needle with 1 cc Syringe ...................................................... 17
Figure 12: Sweat Dispenser .................................................................................. 17
Figure 13: USB Cable ............................................................................................ 17
Figure 14: Electrode Cleaning Pads ...................................................................... 17
1.3 Touchscreen and Operator Interface ............................................................................. 18
Table 2: Main Function Icons................................................................................ 18
Table 3: Settings Icons.......................................................................................... 20
Table 4: Keyboard/Keypad Keys ........................................................................... 21
1.4 Macroduct Advanced Model 3710................................................................................. 22
How It Works........................................................................................................ 22
Error Conditions ................................................................................................... 22
Battery Charge Level Indicator ............................................................................. 23
Electrodes............................................................................................................. 23
1.5 Pilogel Iontophoretic Discs............................................................................................. 24
Efficient Sweat Production................................................................................... 24
Ensuring Patient Safety ........................................................................................ 24
Burns During Iontophoresis.................................................................................. 24
1.6 Macroduct Advanced Sweat Collector ........................................................................... 26
Advantages of the Macroduct Advanced Sweat Collector ................................... 27
Notes Regarding Sweat Rate ................................................................................ 27
Section 2
Macroduct Advanced System Setup
2.1 Unpacking ...................................................................................................................... 28
2.2 Charging the Battery ...................................................................................................... 29
2.3 Powering the Device On/Off .......................................................................................... 31
Powering the Device On....................................................................................... 31
Powering the Device Off ...................................................................................... 31
Automatic Power Off – Inactivity Timeout........................................................... 32
Automatic Power Off – Low Battery..................................................................... 32
2.4 Home Screen.................................................................................................................. 33

4
Section 2
Macroduct Advanced System Setup (continued)
2.5 Settings Screen............................................................................................................... 34
Settings Screen..................................................................................................... 34
Figure 15: Diagram of the Settings Screen ........................................................... 35
System Screen ...................................................................................................... 36
Date/Time Screen................................................................................................. 39
Power Management Screen ................................................................................. 39
Language Screen .................................................................................................. 40
Options Screen ..................................................................................................... 41
Simulated Test...................................................................................................... 43
2.6 The Help Menu............................................................................................................... 44
Section 3
Sweat Induction and Collection
3.1 Preparing for Sweat Induction ....................................................................................... 45
3.2 Inducing Sweat............................................................................................................... 52
3.3 Collecting Sweat............................................................................................................. 55
3.4 Risk of Burns................................................................................................................... 65
Section 4
Sweat Analysis
4.1 An Overview of Sweat Analysis ...................................................................................... 66
Chloride Analysis .................................................................................................. 66
Electrical Conductivity.......................................................................................... 66
Section 5
Troubleshooting and Maintenance
5.1 Troubleshooting ............................................................................................................. 67
Table 5: General Troubleshooting and Diagnosis................................................. 67
Table 6: Error Code Troubleshooting and Diagnosis............................................. 70
Using Functional Test for Troubleshooting .......................................................... 75
Table 7: Functional Test Troubleshooting and Diagnosis ..................................... 76
5.2 Cleaning the Electrodes ................................................................................................. 78
5.3 Cleaning the Device........................................................................................................ 79
5.4 Caring for the Macroduct Straps.................................................................................... 80
5.5 Batteries, Charging, and Calibration .............................................................................. 81
Primary (Non-rechargeable) Battery .................................................................... 81
Secondary (Rechargeable) Battery....................................................................... 81
Charging the Battery ............................................................................................ 82
Battery Calibration ............................................................................................... 83
Replacing the Batteries ........................................................................................ 84
Battery Care ......................................................................................................... 84
5.6 Device Disposal .............................................................................................................. 85
5.7 Shipping or Long-Term Storage of the Device................................................................ 86
Shipping the device to ELITechGroup................................................................... 86
5.8 Customer Service Information ....................................................................................... 87
Appendix A: Pilogel Information .........................................................................................88
Table 8: Critical Components of Pilogel................................................................ 89
Table 9: Hazard and Precautionary Statements................................................... 89
Appendix B: Replacement Parts and Supplies ....................................................................90
Table 10: Replacement Parts and Supplies........................................................... 90
Appendix C: Specifications ............................................................................................................. 91
Table 11: General Specifications, Macroduct Advanced Model 3710 .................. 91
Table 12: Battery Charging Power Supply Specifications ..................................... 92
Appendix D: Procedure for High Skin Resistance ...................................................................... 93
Appendix E: Electromagnetic Compatibility (EMC) ................................................................... 94

5
SECTION 1: INTRODUCTION
1.1 Device Overview
Using This Manual
This manual provides instructions to install, operate, and maintain the Macroduct® Advanced
Sweat Collection System. The manual is an important part of the product. Read it carefully and
completely before setup and first use of the device. Anyone operating the Macroduct Advanced
Sweat Collection System must be thoroughly familiar with the procedures and cautionary
information detailed in this manual before attempting to use this equipment.
If additional accident prevention and environmental protection requirements exist in the country
of operation, this manual must be supplemented by appropriate instructions to ensure
compliance.
Safety Regulations (Macroduct Advanced Model 3710)
Classification
The Macroduct Advanced Model 3710 is classified as Type BF Medical Equipment, Internally
Powered.
This device has been built and tested in accordance with safety regulations under EN 60601-1 3.1
edition. In order to maintain this condition and ensure safe operation, the operator must observe
all the instructions and warnings contained in this manual. For current information about
applicable standards, please refer to the CE Declaration of Conformity included with the
documents shipped with this device.
NOTE: This equipment complies with the following emission and immunity requirements: EN
60601-1-2 and EN 55022/FCC 47 CFR Part 15.
Specification of Safe Use
Using this device in a manner not specified by ELITechGroup Inc. may impair the safety protection
designed into the device and lead to injury. Do not use where flammable anesthetic is present or
in any oxygen-enriched environment.
WARNING!
Do not use this equipment if it is not functioning properly.
Statement of Environmental Limits
This device is tested for safe operation at 15 °C to 30 °C, relative humidity ≤ 85%, non-condensing,
and atmospheric pressure ≥ 79.5 kPa.

6
SECTION 1: INTRODUCTION
1.1 Device Overview
Understanding Warnings
This manual uses three warning levels to alert the operator to important information as shown in
the following examples.
WARNING!
A Warning alerts to the possibility of personal injury, death, or other serious adverse reactions
stemming from the use or misuse of this device or its components.
CAUTION:
A Caution alerts to possible problems with the device associated with its use or misuse. Such
problems include device malfunction, failure, damage, damage to the sample, or damage to
other property. Where applicable, a Caution may include precautions to be taken to avoid the
hazard.
NOTE: A Note reinforces or supplies additional information about a topic.
Specific Warnings
Pay particular attention to the following safety precautions. If these safety precautions are
ignored, personal injury or damage to the device may occur. Each individual precaution is
important.
WARNING!
Due to the possibility of an explosion, never attempt iontophoresis on a patient receiving
oxygen-enriched respiratory therapy in an enclosed space, such as an oxygen tent (nasal
cannula is acceptable). With medical approval, remove the patient from that environment
during iontophoresis.
WARNING!
Do not stimulate or collect sweat from the following sites:
•Head, including forehead (possible burns).
•Trunk (current crossing heart).
•Any area of inflammation (e.g. eczema or rash); serous or bloody discharge
(contamination).
WARNING!
Do not use over areas with metal plates/pins.
WARNING!
Never attempt to reuse single use components/accessories.
WARNING!
Do not use electrodes or pilogel discs that have been altered or appear damaged.

7
SECTION 1: INTRODUCTION
1.1 Device Overview
WARNING!
Consult a physician before performing a test on patients with clinically diagnosed cancer.
WARNING!
Consult a physician before performing a test on patients who have had previous adverse
reactions to electrotherapy.
CAUTION:
Collection of sweat should be carried out at a time when the patient is clinically stable, well-
hydrated, free of acute illness, and not receiving mineralocorticoids.
CAUTION:
Pilogel discs should be refrigerated at 2 °C to 10 °C. DO NOT FREEZE. Never use discs that have
been frozen or that are cracked.
CAUTION:
This equipment has been designed and tested to CISPR 11 Class A and FCC Part 15 Class A. In a
domestic environment it may cause radio interference, in which case, the operator may need to
take measures to mitigate the interference.
CAUTION:
Only spare parts and accessories supplied or specified by ELITechGroup should be used with this
device, including the battery charging power supply and power cord used for charging the
device. Using non-approved parts may affect the performance and safety features of the device.
If the device is used in a manner not specified by ELITechGroup, the protection provided by the
device may be impaired. If in doubt, contact an ELITechGroup representative.
CAUTION:
The USB connection on the device is intended to be used by authorized personnel. For security
purposes, it is recommended to execute a virus/malware scan on any USB flash drives or
computers prior to making connection. As good practice it is recommended to remove any USB
connection prior to performing iontophoresis on a patient.
Contraindications
•Patients with an implanted device, such as a defibrillator, neurostimulator, pacemaker, or ECG
monitor.
•Patients with a history of epilepsy or seizures.
•Patients who are pregnant.
•Patients that have a known sensitivity or allergy to any ingredient.
•Over damaged, denuded skin or other recent scar tissue.
•Patients with Cardiac Conditions or with suspected heart problems.

8
SECTION 1: INTRODUCTION
1.1 Device Overview
Functional Description
Macroduct Advanced Sweat Collection System is intended for laboratory use by qualified
personnel for stimulation and collection of sweat from humans to assist in the laboratory
diagnosis of cystic fibrosis.
The system safely and efficiently accomplishes the stimulation of human sweat through
pilocarpine iontophoresis using the Macroduct Advanced Model 3710. The Macroduct Advanced
Sweat Collector collects a sample of the stimulated sweat. Markings on the tube indicate if a
sufficient sweat rate was achieved during the collection of sweat. The sample can then be
analyzed for indications of cystic fibrosis with the Sweat-Chek™ Sweat Conductivity Analyzer using
the principle of total electrolyte concentration in the sweat sample; or with the ChloroChek®
Chloridometer® using the principle of coulometric titration.
The Macroduct Advanced Sweat Collection System consists of the Macroduct Advanced Model
3710, which is a microprocessor-controlled device powered from a rechargeable lithium-ion
battery, battery charging power supply and cord for charging the battery, electrode cable
assembly, and a kit of single-use supplies (Pilogel discs and collectors). The Macroduct Advanced
Model 3710 automates and controls the sweat collection process used to detect cystic fibrosis. In
that sweat collection process, pilocarpine ions are ‘pushed’ into the sweat glands of the skin by a
small electric current (1.5 mA DC) where they stimulate sweat in the same way as the chemicals
released by the brain to control body heat through sweating on a hot day. After sweating has
been stimulated in a particular area, the electrodes are removed and the skin is cleaned. A plastic
Macroduct Advanced Sweat Collector is strapped to the stimulated area so that the emerging
sweat is directed into plastic tubing coiled on the surface of the collector. The pure sweat
collected in this tubing may be analyzed by methods that are compatible with the sample volume.

9
SECTION 1: INTRODUCTION
1.1 Device Overview
Key Features
•Step-by-step instructions for the sweat stimulation and sweat collection processes.
•Easily-attached electrodes and collector.
•Profiled electrical current to reduce patient discomfort during sweat stimulation.
•Automatic logging of important data during the iontophoresis and sweat collection process.
•Continuously monitors iontophoresis current to maximize patient safety.
•Elliptical-shaped collector, Pilogel discs, and electrodes to better fit small arms (neonate and
toddler-sized arms).
•Complete patient mobility during sweat collection.
•Easily-confirmed sweat rate and total sweat yield gauged by the operator.
•Uncompromised sweat specimen.
•Air-free collector prevents condensate error.
•Negligible (≤ 0.1 microliters per hour) sweat evaporation rate.
•Exportable log files using a micro USB connector.
Intended Use
The Macroduct Advanced Model 3710 Sweat Collection System is intended only for clinical
laboratory use by qualified medical personnel for stimulation and collection of sweat from
humans for analysis for the diagnosis of cystic fibrosis.

10
SECTION 1: INTRODUCTION
1.1 Device Overview
Table 1: Explanation of Symbols
Symbol Description
Classification of degree of protection against electric shock (BF)
Authorized Representative in the European Community
LOT Code
Biological Hazards (Biological Risks)
Reference/Catalog Number (Model Number)
Serial Number
Consult Instructions For Use
Follow Instructions For Use
NOTE: The “Follow Instructions For Use” safety sign is located on the label
on the back side of the device. The designated background color for the
safety sign is blue. The label is printed in black and white only; therefore,
the background color is black instead of blue, but the meaning is the same.
Caution, Consult Accompanying Documents (Attention, see instructions for
use)
General Warning, Risk of Danger
CE Mark, product meets the essential requirements designated in Annex V
of the Medical Device Directive 93/42/EC (MDD).
Do Not Reuse
Do not use if package is damaged
Power (Located next to the power switch.)
DC Positive Polarity (Located by the battery charging power supply input
for charging.)
USB symbol – shows where a USB connection can be made to the device.

11
SECTION 1: INTRODUCTION
1.1 Device Overview
Table 1: Explanation of Symbols (continued)
Symbol Description
Low Battery Symbol
Manufacturer
General Symbol for Recovery, Recyclable
Environment Friendly Use Period
Waste of Electrical and Electronic Equipment (WEEE) Symbol. Under
Directive 2012/19/EU, this equipment cannot be disposed of in a normal
landfill
Use By
Temperature Limitation – indicates high and low limits
Warning, Biological Hazard
Harmful/Irritant
Environmental Hazard
Toxic
12
SECTION 1: INTRODUCTION
1.2 Device Description
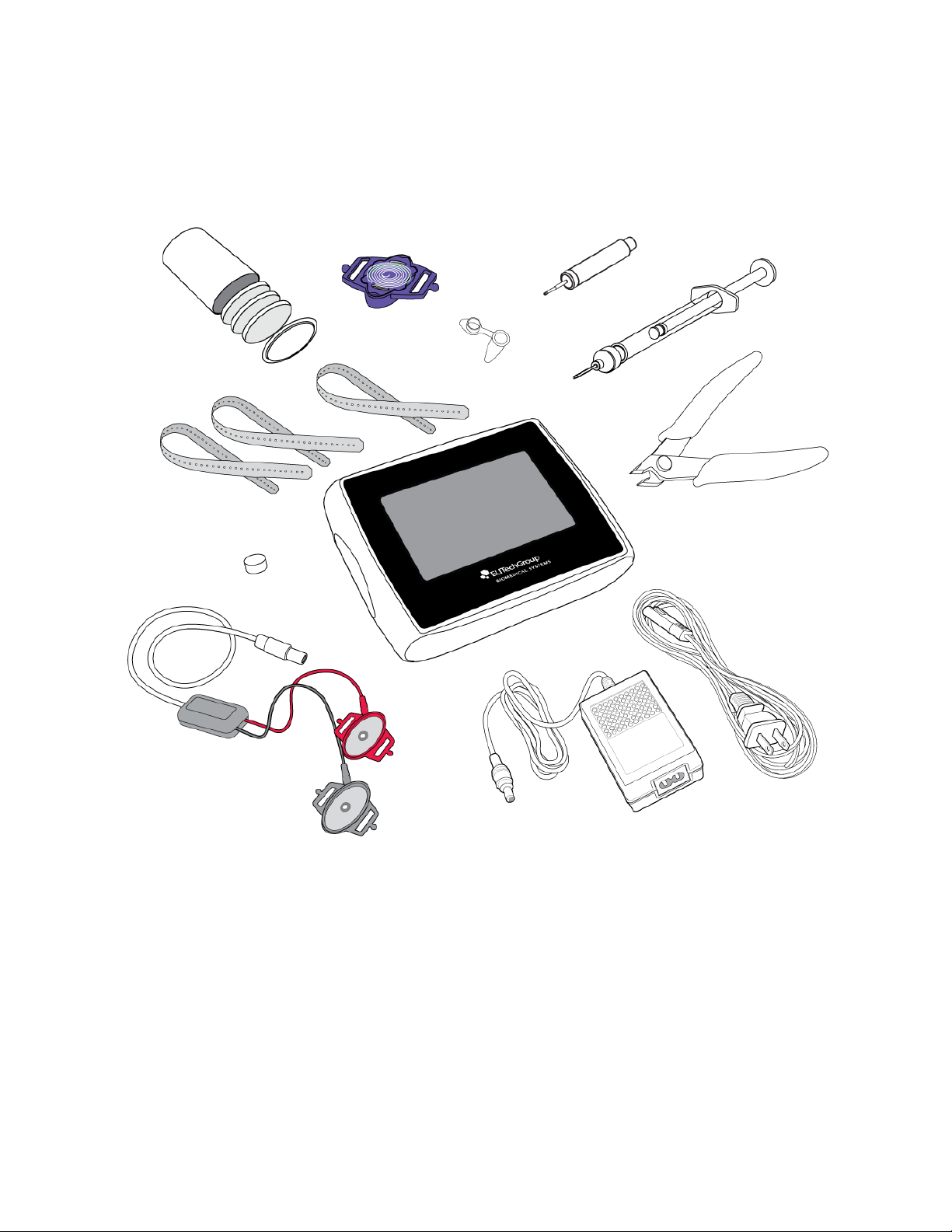
Figure 1: System Components
The following are included in the carrying case:
1. Macroduct Advanced Supply Kit for 6 Sweat Tests
(SS-268)
1a. Pilogel Discs (12)
1b. Macroduct Advanced Sweat Collectors (6)
1c. Small Sealable Containers (6)
2. Straps for electrode and collector, set of 18 (SS-269)
3. Electrode Cleaning Pads, package of 10 (SS-271)
4. Electrode Cable Assembly (AC-203)
5. Sweat Dispenser (RP-065)
6. Syringe (1 cc) with EasyDuct Needle
(AC-193)
7 Nippers (RP-066)
8. Macroduct Advanced Model 3710
9. AC Power Cord
10. Battery Charging Power Supply
11. USB Cable (RP-538) (not shown)
1a. Pilogel Discs
1b. Sweat Collectors
1c. Small Sealable
Containers
5. Sweat Dispenser
6. Syringe (1cc) with EasyDuct
Needle
7. Nippers
9. AC Power Cord
10. Battery Charging Power Supply
4. Electrode Cable Assembly
3. Electrode Cleaning Pads
2. Electrode and Collector Straps
8. Macroduct Advanced
Model 3710

13
SECTION 1: INTRODUCTION
1.2 Device Description
Figure 2: Display
Touchscreen
Operator interaction with the graphical user interface occurs though the touchscreen. Tap the
active area on the display with a finger to select an icon, menu item, or button. The touchscreen
sensitivity allows gloves to be worn during use. To avoid damaging the touchscreen, do not tap it
with anything sharp or apply excessive pressure to it with fingertips. Dragging, swiping, and
pinching gestures are not used.
Display
The display is divided into functional regions for ease of use.
•A task bar is located on the left side of the display. Depending upon the screen, the task
bar allows access to Settings, Home, and context-sensitive Help. The battery charge level
is displayed in the lower left corner.
•A screen title region is located along the top of the display that is used to display the title
of the screen or display information relating to the screen.
•Navigation arrows are located on the bottom left and right side of the display. Depending
upon the screen, these arrows navigate to the next or previous screen or are used to
navigate menus and selection lists.
•The remainder of the display is a graphics/operator input region where process
information is provided along with interaction from the operator when setting device
parameters, entering information, and managing processes.

14
SECTION 1: INTRODUCTION
1.2 Device Description
Figure 3: Top Panel
Item Description
1a. Power Switch
Powers the device ON when held down for 1-2 seconds.
Prompts to power off the device when held down for 2-3 seconds.
Resets the device when held down for 4-5 seconds.
1b. LED – Green/Amber
The power switch contains a two-color LED used to indicate status.
A green LED indicates that the device is powered ON.
When the charging power supply is plugged in:
A flashing amber LED indicates that the battery is being charged.
A solid amber LED indicates that the battery is fully charged.
2. Electrode Connector
The electrode connector is a 6-pin push-pull locking medical connector
that mates with the electrode cable assembly.
3. USB Connector
The micro USB connector is used when connecting the device to a
computer or USB flash drive.
4. Charging Connector
The battery charging power supply connects to the circular DC power
charging connector to charge the battery. When connected, the
iontophoresis-related circuits are disabled and access to the user
interface is not allowed except for the battery charging screen.
1. Power Switch/LED
3. USB Connector
4. Charging Connector
2. Electrode Connector

15
SECTION 1: INTRODUCTION
1.2 Device Description
Figure 4: Model/Serial Number Identification label
The following label is located on the back of the device:
Figure 5: Electrode Cable Assembly
The electrode cable assembly connects to the device at the electrode
connector on the top panel.
Both electrodes, one red for the anode (positive) and one black for the
cathode (negative), have a stainless-steel disc as the electrode plate. In
the center of each electrode is a pin for detecting the Pilogel disc. The
electrodes provide current from the device through the Pilogel discs to
the patient skin during iontophoresis.
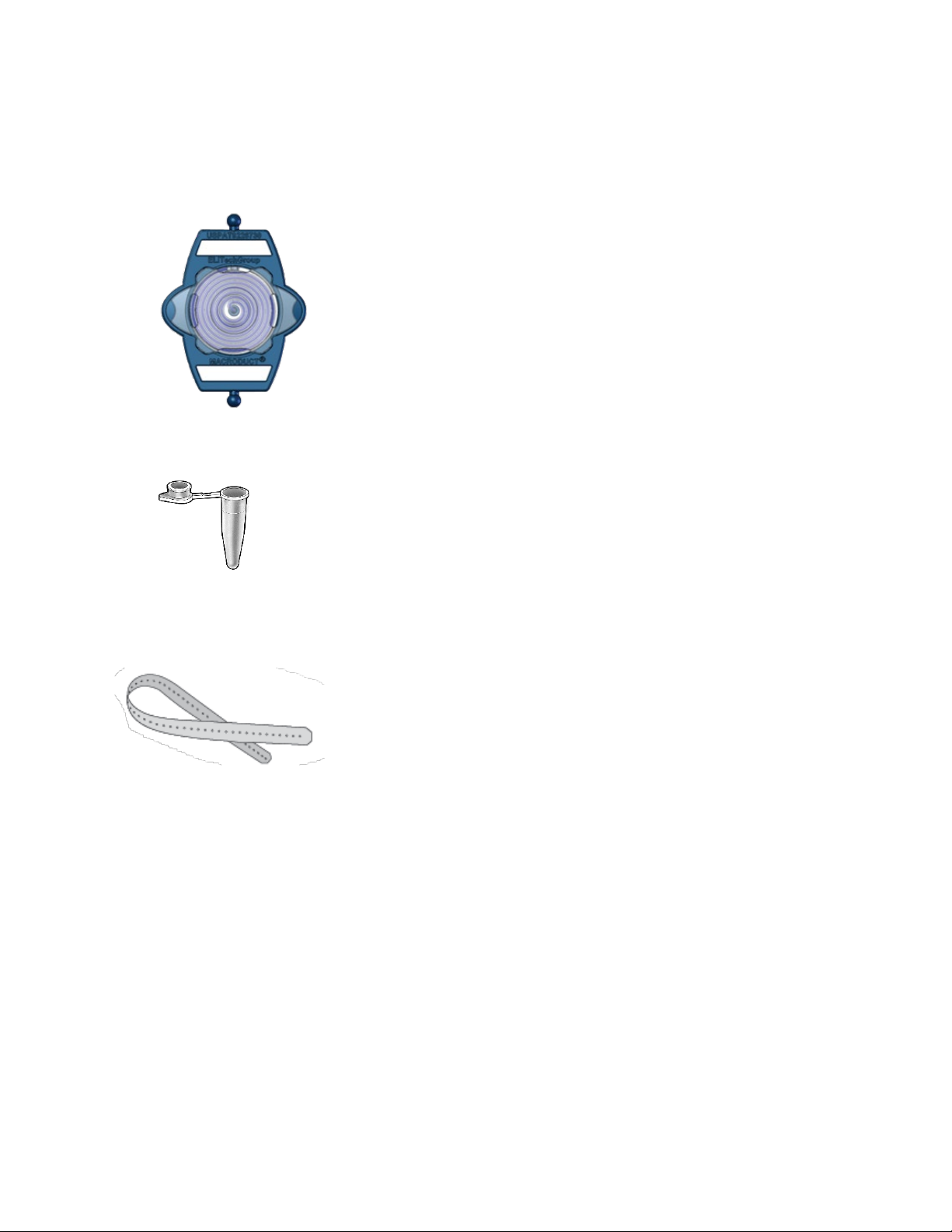
Figure 6: Pilogel Discs
Elliptical Pilogel discs are included in the SS-268 Macroduct Advanced
Supply Kit. The discs are approximately 6 mm (0.25 inch) thick and sized
to fit snugly into the standard recessed electrodes. They are supplied in a
resealable vial containing 12 discs each and are intended for single use
(sufficient for six iontophoretic sweat stimulations). Discs are to be used
in both positive (red) and negative (black) electrodes (applied parts).
Sweat stimulation occurs under the positive (red) electrode, while the
negative electrode completes the electrical circuit.
16
SECTION 1: INTRODUCTION
1.2 Device Description
Figure 7: Macroduct Advanced Sweat Collector
The Macroduct Advanced Sweat collectors are used to collect the sweat
after iontophoresis. Six individually packaged collectors are included in
the SS-268 Macroduct Advanced Supply Kit and are intended for single
use.
Figure 8: Small Sealable Containers
The small sealable containers (200 µL micro-centrifuge tubes) are
included in the SS-268 Macroduct Advanced Supply Kit and are used to
store sweat samples for up to 72 hours when properly used. The small
sealable containers come packaged in a set of six (enough for six tests)
and are intended for single use.
Figure 9: Collector and Electrode Straps
The Macroduct Advanced straps are used to attach the electrodes and
collector to a patient (applied part). The straps are disposable or can be
re-used (see Section 5.4 for cleaning/disinfecting information) and come
packaged in a set of 18 (enough for six tests, one strap for each
electrode and one strap for the collector). The straps were designed for
ease of use and can accommodate a wide range of limb sizes. The non-
allergenic straps are a non-latex thermoplastic elastomer material.
17
SECTION 1: INTRODUCTION
1.2 Device Description
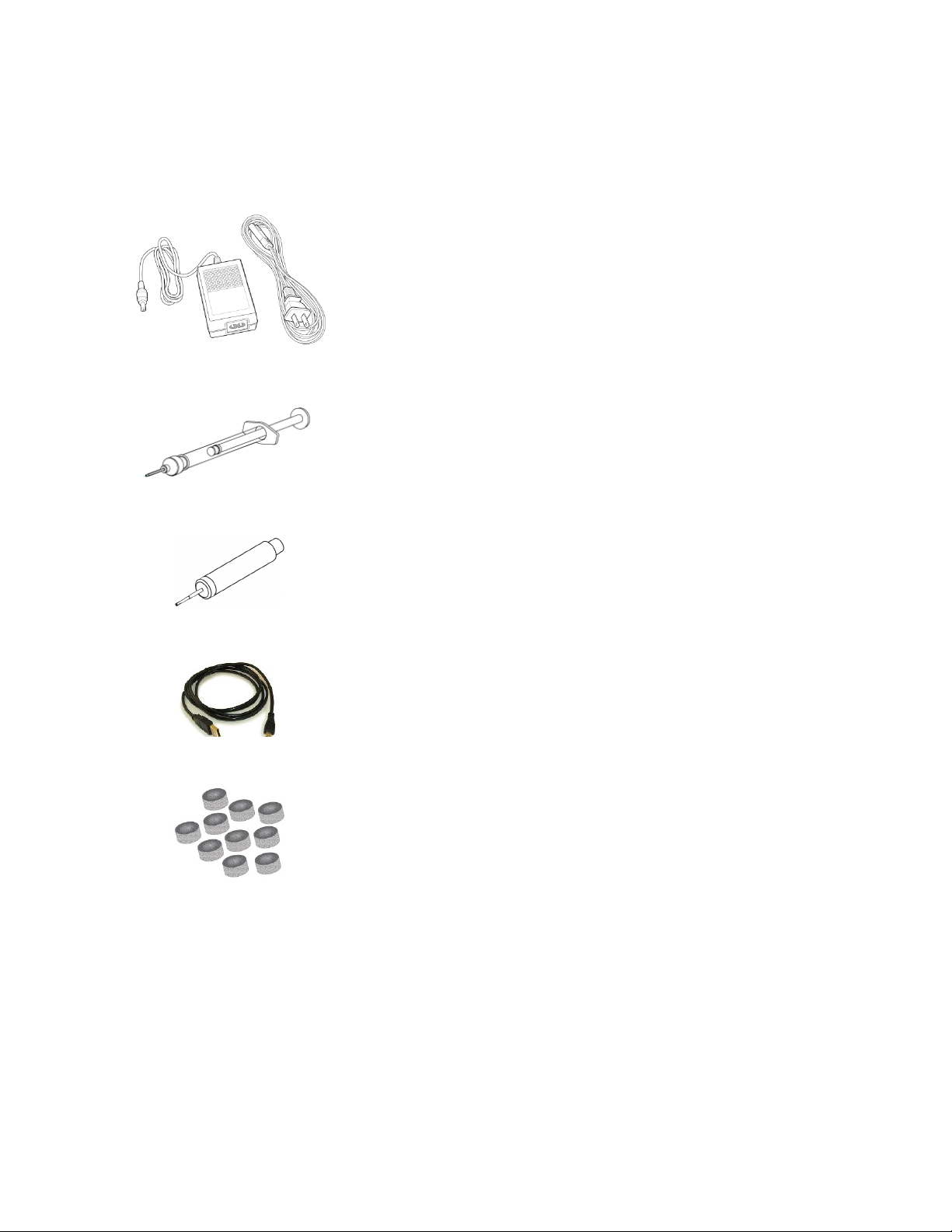
Figure 10: Battery Charging Power Supply and AC Power Cord for Battery Charging
The Macroduct Advanced Sweat Collection System includes a universal
input battery charging power supply and AC power cord (120 V cord
shown).
Figure 11: EasyDuct Needle with 1 cc Syringe
The syringe and EasyDuct needle are used to harvest a sweat sample.
The EasyDuct needle is specially designed for easy insertion into the
collector tubing.
Figure 12: Sweat Dispenser
The sweat dispenser is an optional tool used to harvest and store a
sweat sample in the small sealable containers. The sweat dispenser uses
a reduced-end blunt needle for easy insertion into the collector tubing.
Figure 13: USB Cable
The USB cable is a 6-foot long USB A Male to USB Micro B Male cable
used to interface the Macroduct Advanced Model 3710 to a computer
USB port.
Figure 14: Electrode Cleaning Pads
The electrode cleaning pads are packaged in a pack of 10 and are used to
clean and buff the electrodes. They are a mild abrasive pad, which
provide gentle, yet thorough cleaning of the electrodes. The pads are
sized to easily fit within the electrodes and can be used with a fingertip.

18
SECTION 1: INTRODUCTION
1.3 Touchscreen and Operator Interface
The operator controls all device functions from the interactive touchscreen display.
Table 2: Main Function Icons
Icon Name Description
Home Returns the operator to the Home screen.
Help Accesses context-sensitive help menu.
Settings Accesses the Settings screen.
Battery Indicator Displays the amount of charge remaining in the battery.
Battery Low
Indicator Indicates that the battery is low and should be recharged.
Cancel Cancels a process or function.
Forward Arrow Advances to the next screen.
Back Arrow Returns to the previous screen.
Selected Shows that the associated option is selected.
Unselected Shows that the associated option is not selected.
Begin Begins the step-by-step iontophoresis setup procedure from
the Home screen.
Start Iontophoresis Starts the iontophoresis sweat stimulation process.
Table of contents