EMD Serono RebiSmart 3.0 User manual

Model: 3.0
RebiSmart® autoinjector delivers a
pre-set dose of Rebif®.
Instructions for Use
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

Introduction to RebiSmart®.............................................................6
Section 1.1 About the device .......................................................................................................... 8
Section 1.2 Indications and contraindications........................................................................... 9
Section 1.3 Safety precautions ....................................................................................................... 10
Section 1.4 Device and supplies ..................................................................................................... 12
Getting started ..................................................................................20
Section 2.1 Charging the device..................................................................................................... 22
Section 2.2 Enter device settings................................................................................................... 24
Section 2.3 Inserting cartridge........................................................................................................ 27
Contents
1
2
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

Injecting medication with RebiSmart®........................................30
Section 3.1 Prepare for injection.................................................................................................... 32
Section 3.2 Attach needle ................................................................................................................ 36
Section 3.3 Administer the injection............................................................................................. 38
Section 3.4 Detach needle and confirm injection ..................................................................... 40
Caring for the device.........................................................................42
Section 4.1 Storing device and cartridges................................................................................... 44
Section 4.2 Taking care of your device......................................................................................... 45
Section 4.3 Changing cartridge ...................................................................................................... 46
Section 4.4 Replacing device........................................................................................................... 50
Section 4.5 Travel with device ........................................................................................................ 51
4
5
3
4
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

5
6
Settings.................................................................................................52
Section 5.1 Menu overview.............................................................................................................. 54
Section 5.2 History ............................................................................................................................. 55
Section 5.3 Injection settings.......................................................................................................... 57
Section 5.4 Device settings.............................................................................................................. 62
Section 5.5 Battery status................................................................................................................ 68
Troubleshooting..................................................................................70
Section 6.1 Interrupted injection ................................................................................................... 72
Section 6.2 Dropped device.............................................................................................................. 74
Section 6.3 Warning and information messages ...................................................................... 76
Section 6.4 Frequently asked questions ...................................................................................... 84
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

4
7
8
Additional information for doctors or nurses.............................94
Section 7.1 Complete first time setup wizard ............................................................................ 96
Section 7.2 Clinical setup................................................................................................................. 100
Technical specifications and appendix.........................................104
Section 8.1 Technical data ............................................................................................................... 106
Section 8.2 Explanation of symbols............................................................................................... 108
Section 8.3 Electromagnetic specifications ................................................................................ 111
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

Introduction to
RebiSmart®
Section 1
This section introduces you to
RebiSmart® and provides important
safety information about using
RebiSmart® to deliver Rebif® medication.
1.1 About the device 8
1.2 Indications and contraindications 9
1.3 Safety precautions 10
1.4 Device and supplies 12
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP
8
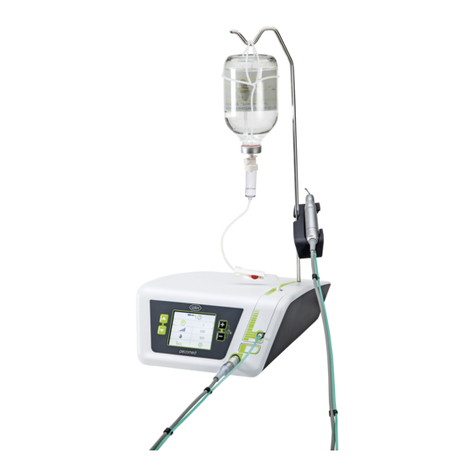
RebiSmart® is an electromechanical
autoinjector which delivers a pre-set dose of
Rebif® (interferon beta-1a for injection).
• RebiSmart® keeps track of your injections.
You can review up to 12 months of
injections in the History screen (from the
current month and previous 11 months).
• 48 hours are recommended between
injections. To prevent from injecting too
often, RebiSmart® warns you if a new
injection is attempted less than 48 hours
after the previous one.
• RebiSmart® contains no harmful or toxic
materials.
Section 1.1 About the device
• RebiSmart® will not store or transfer any
personal information.
• If you have any questions, talk to your
doctor or nurse.
• By turning on the “Data transfer” feature
in RebiSmart®, you can view your injection
history on a mobile application accessible
by you, if it is available in your country.
Data means device injection data and
technical log. It does not contain any
personal identifiable information. It is
anonymous.
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

9
1.2 Indications and contraindications
Section 1.2 Indications and contraindications
Indications for use:
RebiSmart® is a reusable, electromechanical
software-controlled autoinjector intended for
subcutaneous (under the skin) injection of
Rebif® medication.
RebiSmart® is indicated for use by patients
treated with interferon beta-1a (Rebif®).
The patient population is comprised
predominantly of adults (18+ years) but also
adolescents (12-17 years). Intended users
of the product also include the patient’s
caregivers, nurse, doctor and medical support
sta.
RebiSmart® facilitates the administration
of Rebif®, including self-administration by
patients at home. In addition, RebiSmart®
supports adherence management by recording
the dose history and providing adjustable
comfort settings.
Contraindications for use:
You should not use RebiSmart® if you
have severe dexterity, visual, upper
extremity or cognitive neurological
deficits.
Your doctor or nurse will advise if you
need support from a caregiver for your
injections.
For contraindications related to Rebif®
medication, please read the Rebif® Patient
Information Leaflet.
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

10
This section provides general precautions related to using RebiSmart®. Additional warnings and
cautions related to specific instructions are highlighted throughout this booklet. If you have any
questions after reading this section, do not hesitate to ask your doctor or nurse.
• Carefully read all instructions in this
booklet before using RebiSmart® and
follow these Instructions for Use while
using RebiSmart®. These Instructions
for Use provide important safety
information about RebiSmart®.
• DO NOT discard these Instructions for
Use. Keep this booklet in a safe place for
future reference.
• You must be trained by a qualified
doctor or nurse before using RebiSmart®.
If you have not received training, please
contact your doctor or nurse.
• RebiSmart® is a personal device for
use by only one patient. DO NOT share
RebiSmart® with anyone else. To avoid
risks of injury, including the transmission
of infectious bloodborne diseases.
Section 1.3 Safety precautions
Before you proceed, review these general warning and cautions.
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

11
1.3 Safety precautions
• DO NOT change the injection settings on
RebiSmart® until you have discussed it
with your doctor or nurse.
• Take care not to drop RebiSmart®.
RebiSmart® should be handled with care.
• DO NOT use RebiSmart® if it is not
working properly.
• DO NOT attempt to modify, or repair the
device, or an incorrect dose might be
delivered. Contact your doctor or nurse in
case of device malfunction.
• DO NOT insert any foreign object into the
needle cavity.
• Use Rebif® in accordance with the Patient
Information Leaflet.
• DO NOT load non-Rebif® cartridges into
RebiSmart®. Only use original Rebif®
cartridges.
• DO NOT use a cartridge if the label is
missing or damaged.
• To avoid administration of non-sterile
medication:
DO NOT use a Rebif® cartridge if it
shows any signs of damage,
DO NOT reuse a cartridge rejected by
RebiSmart®
• To avoid risks of injury caused by a
broken cartridge, DO NOT try to remove
broken glass from your RebiSmart®.
• DO NOT touch or use the battery charger
if it shows signs of damage or is broken.
• Make sure the charging cable does not
cause a hazard of tripping or other
injury.
• DO NOT allow children to play with the
charging cable.
• Report any serious incidents involving
your RebiSmart® to the RebiSmart®
local representative and your national
competent authority for medical devices.
General warning and cautions (continued)
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

12
Items provided in device packaging:
* Number of plug adapters may vary (included for dierent regions)
** Number of Instructions for Use booklets may vary (included for dierent languages).
A distributor list is part of device packaging.
NOTE: Contact your doctor or nurse for information on the supply of medication
cartridges and needles, or if you need replacements for RebiSmart® or any supplies.
1 RebiSmart®
autoinjector
1 Storage box1 Battery charger*1 Battery Instructions for Use**
Section 1.4 Device and supplies
i
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP
13
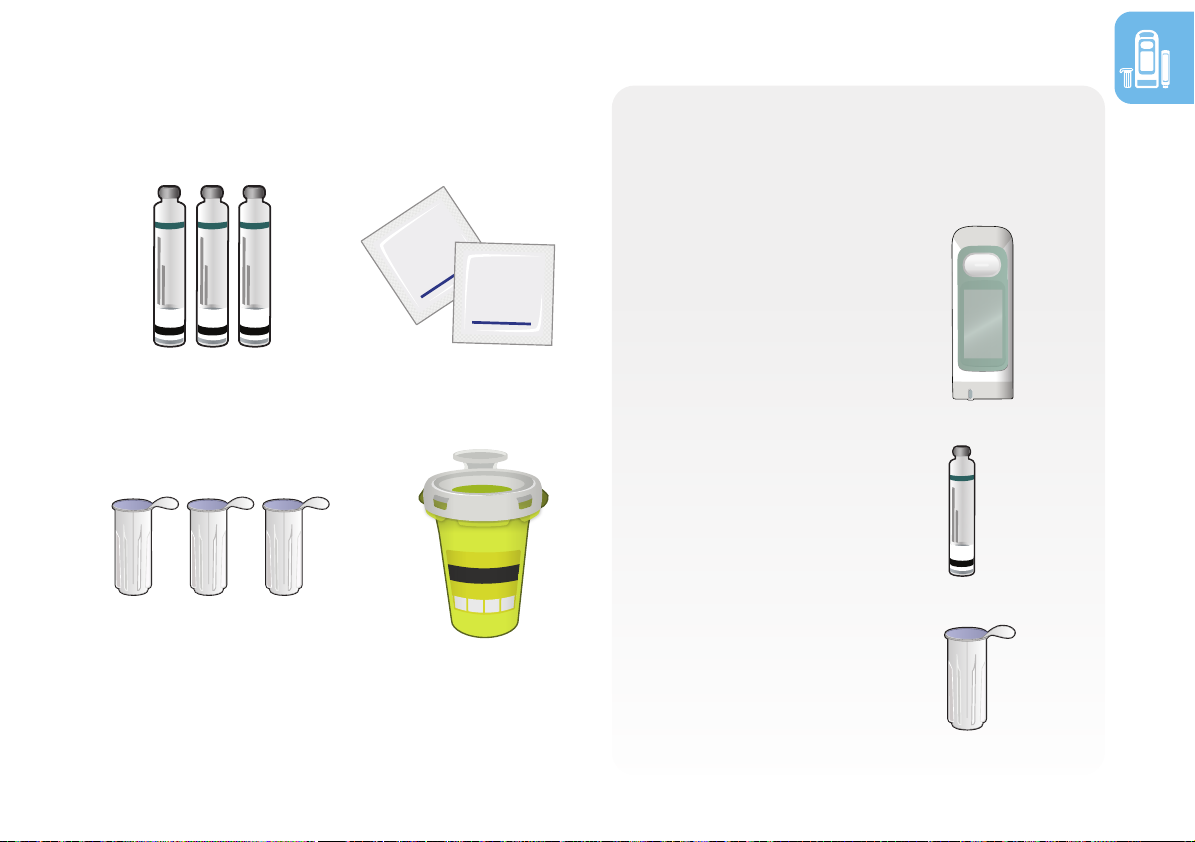
• RebiSmart®
autoinjector
delivers Rebif®
medication
• Rebif® cartridge
containing Rebif®
medication
• Serofine® needle
(29G) for every
injection
The following pages include details about
each of the main components:
Other supplies needed:
Biohazard (sharps)
container
Alcohol wipes
Rebif® cartridges
Serofine® needles
(29G)
1.4 Device and supplies
Not all cartridges, needles, or supplies are
approved or available for use in all countries.
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

14
Section 1.4 Device and supplies (continued)
RebiSmart® autoinjector
RebiSmart® is an electromechanical device that automatically injects the set dose of medication
through single-use needles it inserts and retracts on each injection.
Injection button
Front cover
Touch screen
• The skin sensors around the needle cavity detect if
the device is positioned correctly onto your skin.
• You can remove the battery to charge it or charge
the battery while it is in RebiSmart®.
• Store RebiSmart® in the storage box to keep it clean
and avoid contamination from food and dust.
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP
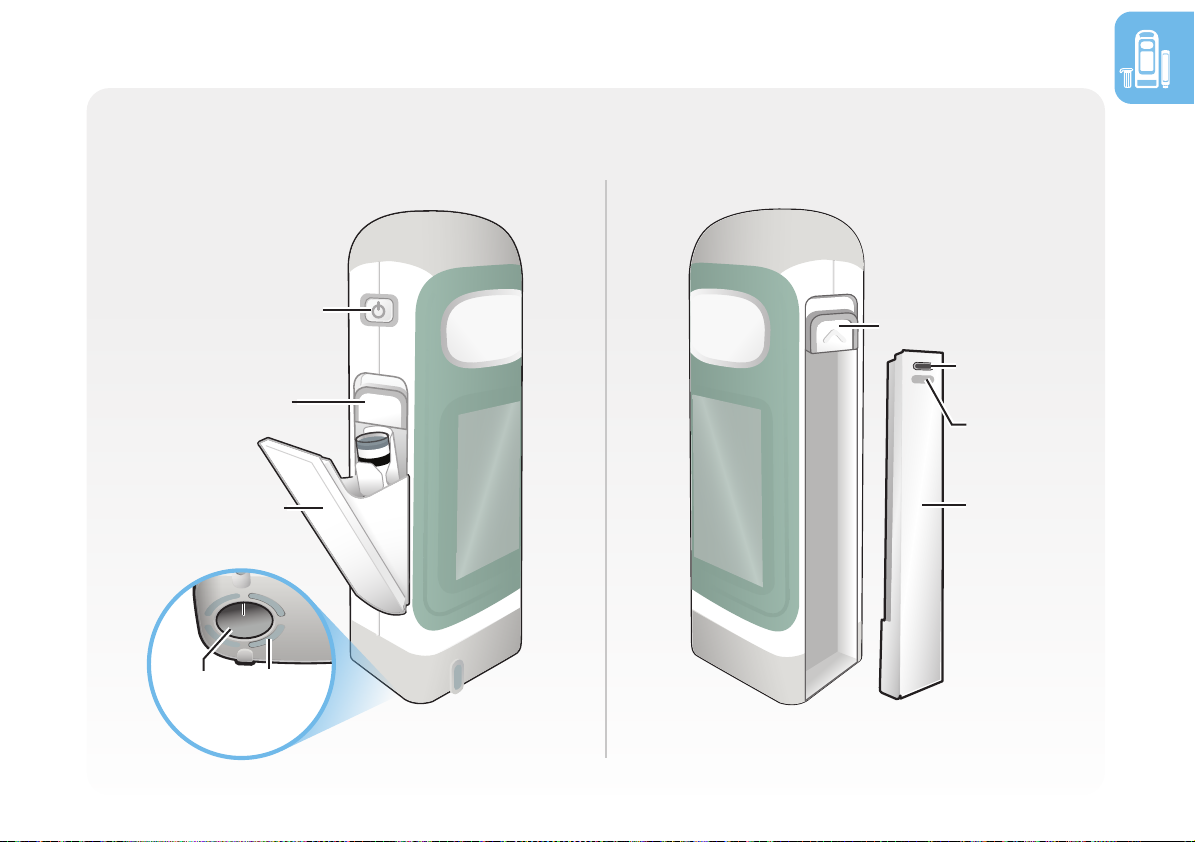
15
On/O button
Cartridge
door
Cartridge
door latch
Needle
cavity
Skin
sensor
Side views
Battery
Status
light
Battery
latch
USB-C
port
1.4 Device and supplies
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

16
Touch screen
The touch screen provides current information, settings, and
instructions on how to administer an injection. You can press
buttons, or swipe up and down on lists to navigate the screens and
make selections.
You can change the colours of the screen (the screen colours in this
booklet might be dierent then the colours you have chosen).
Buttons on the home screen:
Menu button: provides access to the Injection settings,
Clinical setup, Device settings, and History (overview of
previous injections) menus
On-screen injection button: begins the guided injection
process
Last injection: shows how many hours ago you administered
the previous injection
Doses left: shows how many doses are left in the Rebif®
cartridge
Section 1.4 Device and supplies (continued)
Press buttons
Swipe
scroll
bar
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP
17
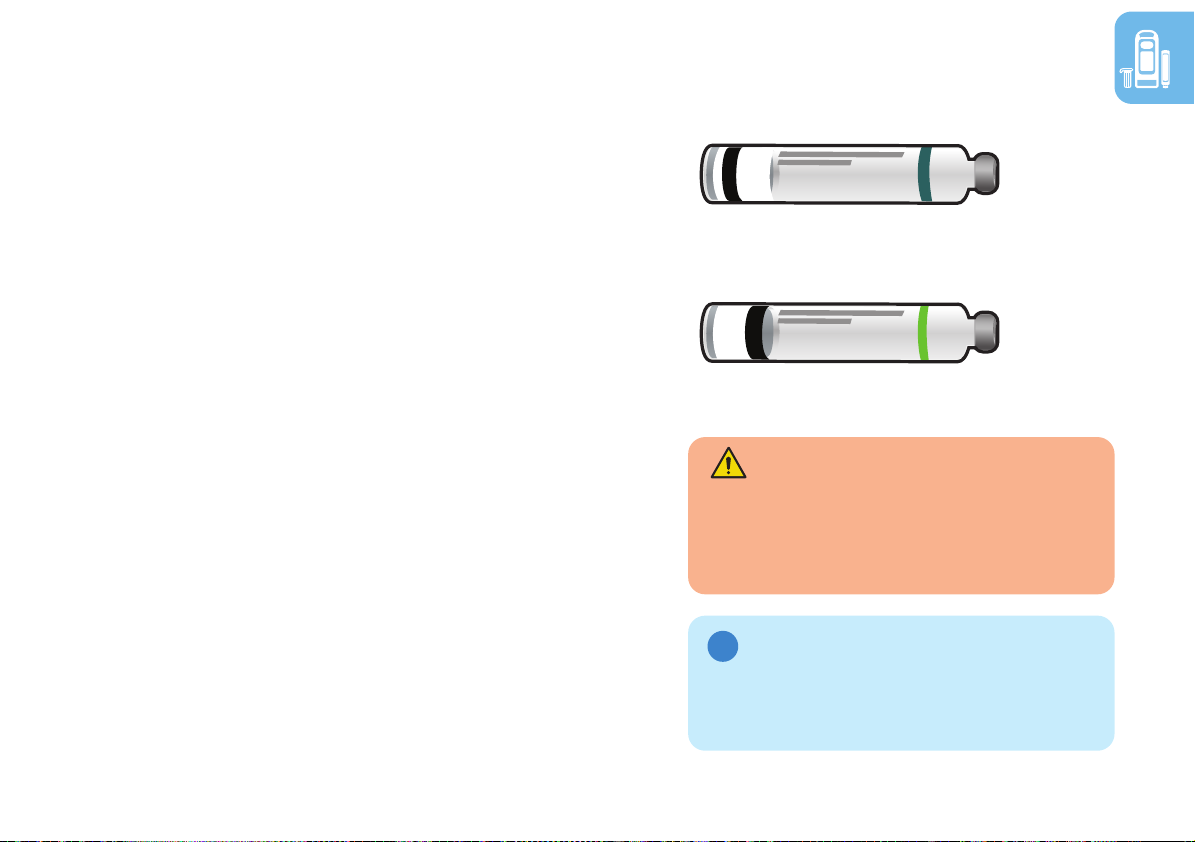
Rebif® cartridges
There are two Rebif® cartridge types compatible
with RebiSmart®:
• 132 µg (microgram, mcg), i.e., 3 doses of
44 µg (concentration: 44 µg/0.5 mL)
• 66 µg, i.e., 3 doses of 22 µg
(concentration: 22 µg/0.5 mL)
Carefully check the Rebif® medication packaging
and cartridge label to ensure that you have the
correct medication and cartridge type.
Rebif® cartridges have a 28-day expiration after
first use. Discard the cartridge if 28 days have
passed since the first injection.
Detailed medication information and the
administration of Rebif® is provided in the Rebif®
Patient Information Leaflet.
WARNING: Make sure to use
the correct Rebif® cartridge with
your RebiSmart®. Using the wrong
cartridge can cause serious harm.
NOTE: DO NOT remove cartridge
label. Otherwise RebiSmart® will reject
the cartridge.
132 µg (44 µg/0.5 mL)
1.4 Device and supplies
i
66 µg (22 µg/0.5 mL)
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP
18
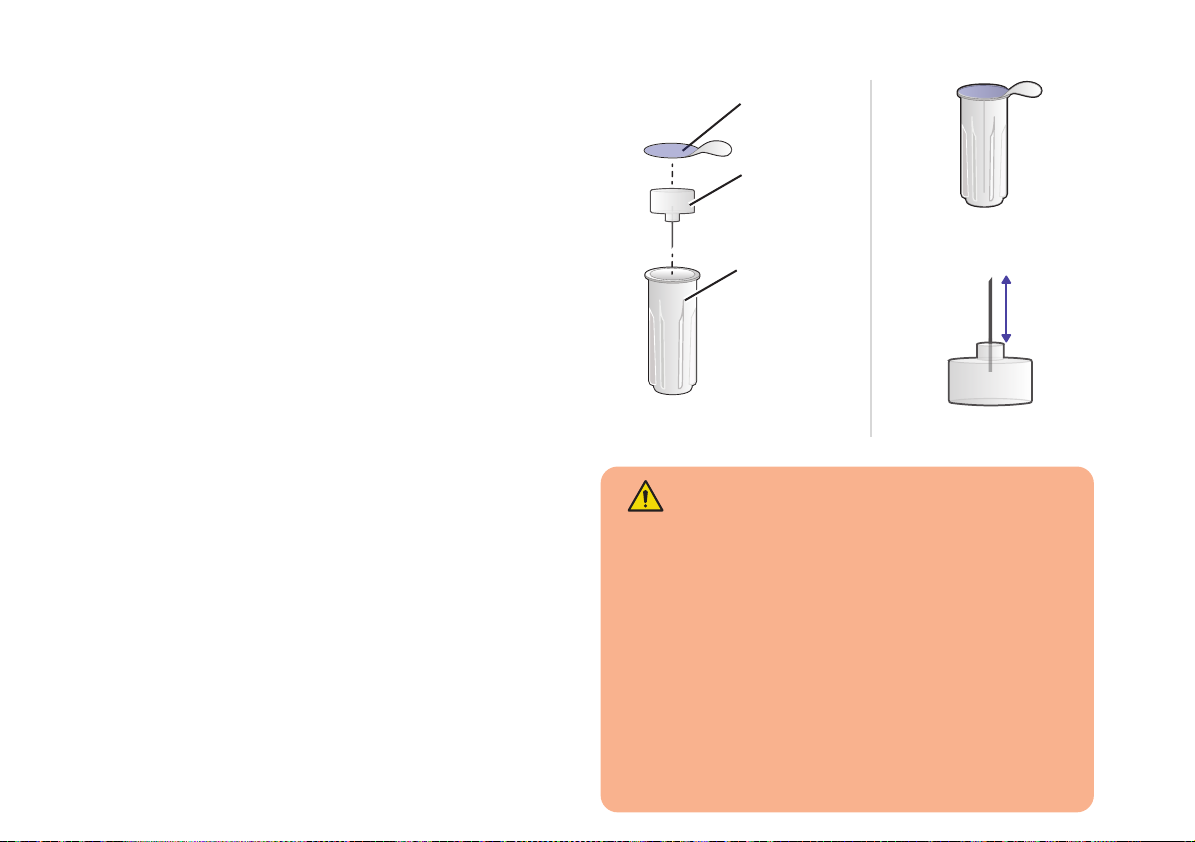
Serofine® needles
There is one Serofine® needle type
compatible with RebiSmart®:
• 29G × 1/2”
(thickness: 0.33 mm × length: 12 mm )
Carefully check the size (or gauge “G”) on
the needle carton and the sterility seal of
each individual needle to ensure if you
have the correct needle, prescribed by your
doctor or nurse.
Dispose of used needles safely in a
biohazard (sharps) container after each
injection.
Serofine®needle
29G needle
12 mm
WARNING
• DO NOT re-use needles to avoid risks of
infection.
• RebiSmart® must be used with
Serofine®single-use, disposable, sterile
needles only.
• Keep needles away from children 3 years
old and younger to avoid risks of needle
stick injury of third party.
Sterility
seal
Section 1.4 Device and supplies (continued)
Needle
Needle
cap
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

19
1.4 Device and supplies
WARNING: DO NOT insert your
hands into the biohazard (sharps)
container for any reason to avoid needle
stick injury.
Discard supplies and device
Use a sharps container to dispose of used
needles after each injection and to dispose of
used cartridges.
Dispose of needles in the sharps container if
the expiration date has passed.
Refer to Rebif® Patient Information Leaflet for
cartridge disposal. Dispose of cartridges in the
sharps container if:
• it is cracked
• the expiration date has passed
• the medication is discolored or contains
particles.
When your sharps container is full, dispose of
it in accordance with your local community
guidelines. If you are not sure about the local
disposal guidelines, ask your doctor or nurse.
If you no longer use RebiSmart®, dispose of
it in accordance with your local regulation.
Contact your doctor or nurse for more
information.
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP

Section 2
2.1 Charging the device 22
2.2 Enter device settings 24
2.3 Inserting cartridge 27
This section provides instructions
for setting up the device before
administering a first injection.
Getting
started
Classification: Internal
Infotable_CHD_V01
ARTWORK INFORMATION
REF: MDT-1016_2022_11-EN-US-V01
TYPE OF COMPONENT: IFU_IFU
PRODUCT: REBISMART E. INJECTOR
VARIANT 3.0
STRENGTH N/A
PACK SIZE 1
MARKET / LANGUAGE: EN-US
TECHNICAL DRAWING: n/a
ARTWORK DIMENSIONS: 210x148 mm
COLOURS:
CMYK
TECHNICAL INFO:
TRACEABILITY
Version Date Designer
01 10 NOV 2022 TP
Table of contents
Other EMD Serono Medical Equipment manuals