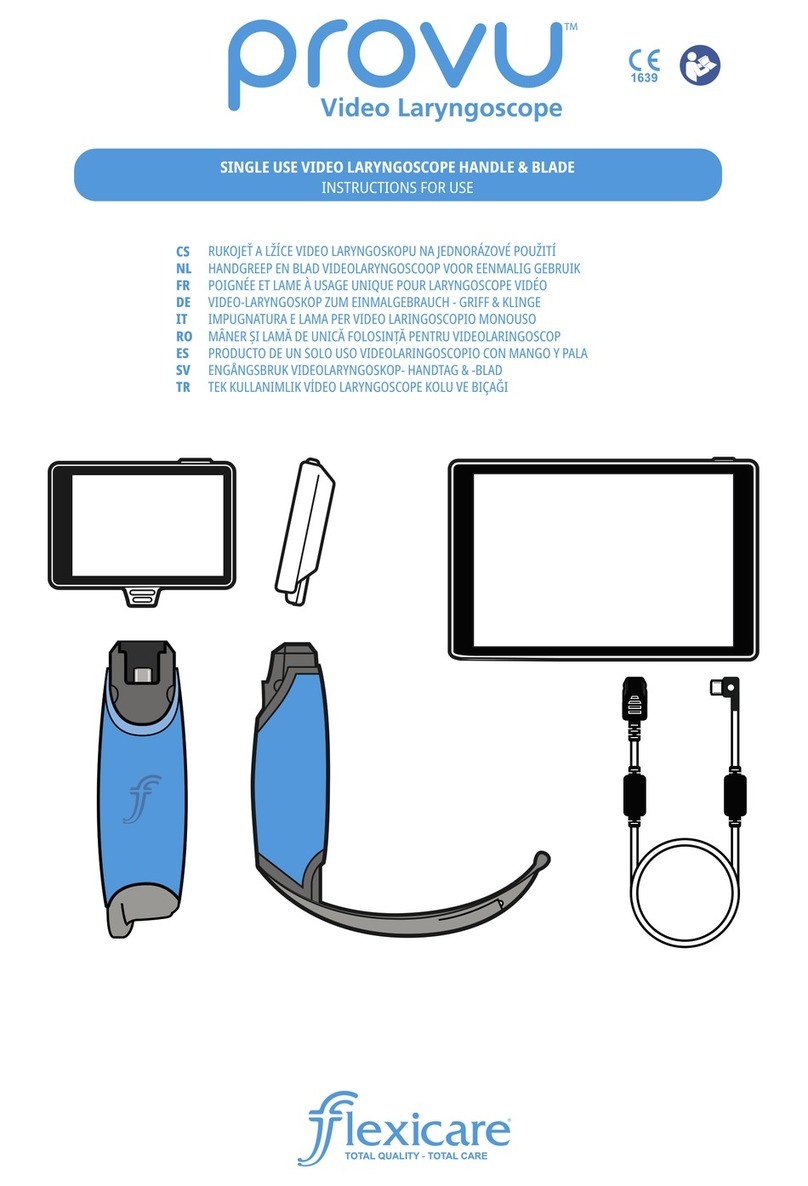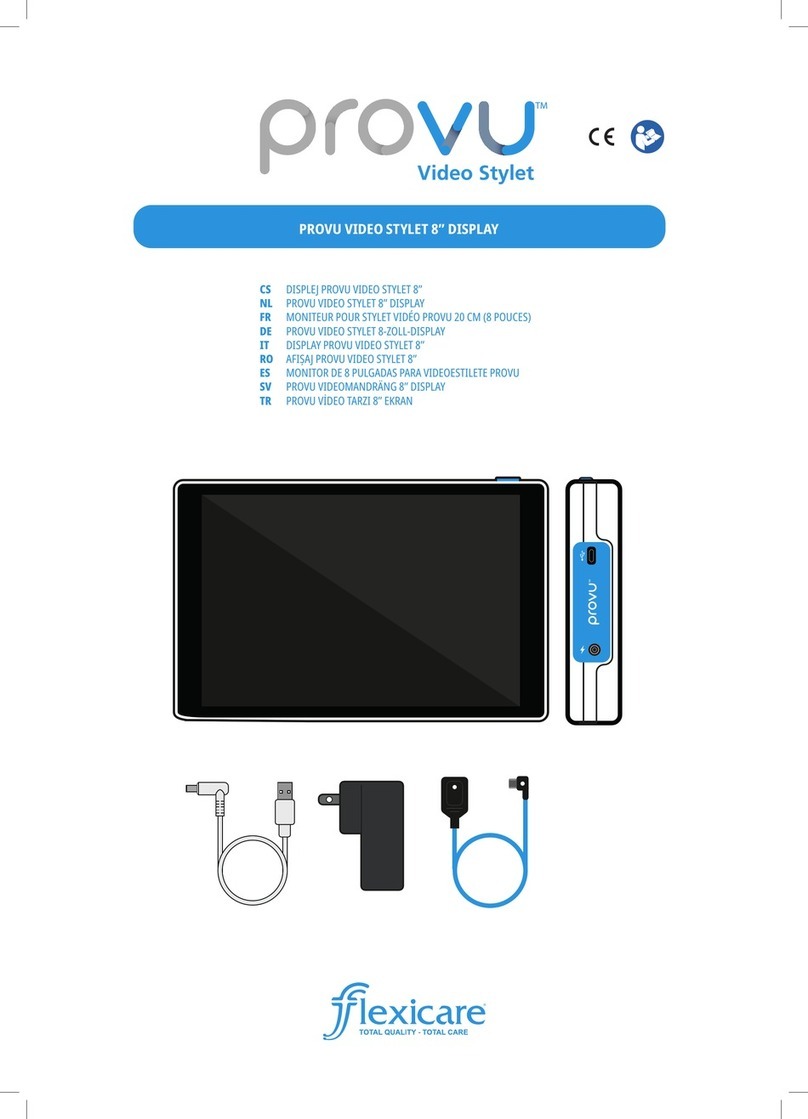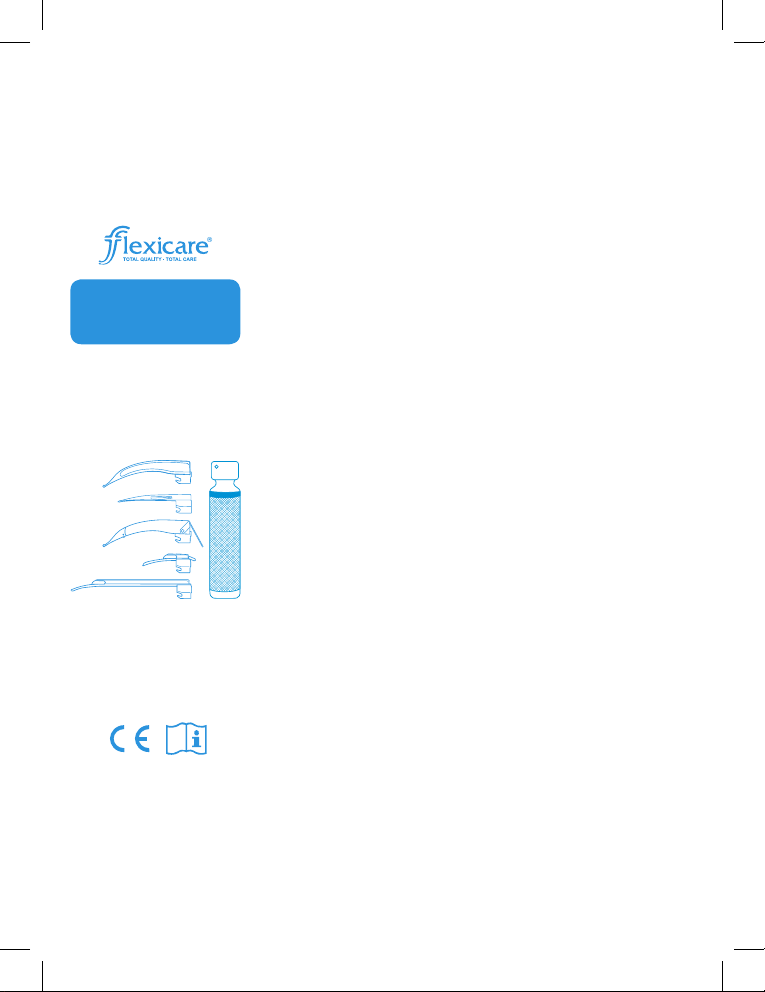
INSTRUCTIONS AND GUIDELINES
FOR REPROCESSING FLEXICARE
REUSABLE LARYNGOSCOPE
HANDLES & BLADES:
Reprocessing should be undertaken by
specialists with the necessary training
and understanding of the process and
equipment. These instructions are
intended for use by these specialists and
should be read in conjunction with all
other relevant manufacturer’s instructions,
hospital policies, local guidelines/
regulations.
Immediately after use and before
cleaning, remove the batteries and light
source from the laryngoscope.
To remove from a conventional blade,
unscrew the bulb from the tting.
To remove the light source from a bre
optic handle, unscrew the knurled
housing from the underside of the blade
block.
Remove the blade from the handle and
immerse both soiled components in a
holding solution of disinfectant/enzyme
solution.
Precautions
- Use pre-approved packaging
designed for use with your
sterilisation equipment.
- Follow all manufacturer’s instructions.
- Prior to sterilisation, ensure the
handle and blade is completely dry
and visually clean. If contaminants are
visible, repeat the decontamination/
cleaning procedure.
- Always wear appropriate PPE and
handle devices and materials with
care.
- If the device will be unused for
prolonged periods, remove the
batteries prior to storing.
- Always check the condition of the
batteries by switching on the light
source before commencing clinical
procedure.
Warnings and Cautions
- Avoid using mineral acids and
abrasive agents.
- DO NOT use sterilants with caustic
ingredients, such as surgical scrub
solutions, peroxide solutions, bleaches,
or povidone-iodine solutions.
- DO NOT sterilise along with sub-
standard stainless steel instruments
as this may cause severe damage.
- No part of the process should
exceed 138°C.
- Ultrasonic cleaning and ash
autoclaving is not recommended.
Decontamination/Cleaning
Clean the removed light source using
a cloth dampened with 70% isopropyl
alcohol.
Disassembled handles and blades should
be decontaminated as soon as possible
after use by either:
A. Automated washer/disinfector:
Use only validated washer/disinfectors
and cleaning agents. Follow the washer/
disinfector manufacturer’s instructions for
use, warning and recommended cycles.
B. Manual processing:
Use dedicated equipment and Personal
Protective Equipment (PPE).
Fully submerge the laryngoscope handle/
blade in a compatible dilute detergent
solution. Brush/scrub/wipe all surfaces to
remove all visual contamination.
Remove from the solution and allow to
drain.
Rinse thoroughly with clean water and
allow to drain before drying.
Sterilisation
Flexicare recommends reprocessing
laryngoscopes blades and handles
through sterilisation. Follow instructions
and warnings as issued by manufacturers
of any decontaminates, disinfectants and
cleaning agents used.
Please adhere to the following
laryngoscope autoclave parameters:
Temp: 134 -138°C, Pressure: 2.25bar,
Cycle time: 3 minutes
Other forms of sterilisation may be
available such as an Orthophthalaldehyde
(OPA) soak and Ethylene Oxide (≤65°C).
These are suitable only if used in
accordance with the manufacturer’s
instructions. If in doubt, consult the
manufacturer.
REUSABLE LARYNGOSCOPE
HANDLES AND BLADES
120mm
160mm