Ferraris Respiratory eSense PiKo User manual

USER’S MANUAL
eSense™ PiKo
®
Ferraris Respiratory, Inc.
901 Front Street, Louisville, CO 80027, USA
Tel: 303-666-5555, Fax: 303-666-5588
Website: www.ferrarisrespiratory.com
FCC ID: TQTPIKOBLI
eSenseTM Piko®
This device complies with Part 15 of the FCC Rules.
Operation is subject to the following two conditions: (1) this device
may not cause harmful interference and (2) this device must accept
any interference received, including interference that may cause
undesired operation.
Changes or modifications not expressly approved by Ferraris
Respiratory, Inc. could void the user’s authority to operate the
eSense
TM
Piko
®

eSense
TM
Piko
®
User’s Manual
Intended Use
The eSenseTM Piko®is used for monitoring respiratory
conditions such as COPD and Asthma.
The eSenseTM Piko®measures the Peak Expiratory Flow
(PEF) and the Forced
Expiratory Volume in the first second of expiration (FEV1).
It displays the test results and also shows the respective
color-zone relative to the patient’s PEF or FEV1 reference
values.
In addition, the blow quality is evaluated to notify the patient
to repeat the test in case of a cough or any suspect blow.
PEF - the fastest speed a person can blow air out of their
lungs.
FEV1 - the amount of air blown out in the first second of a
forced exhalation.
Color Zones - display the test results in a “traffic light” color
format relative to the patient’s reference PEF or FEV1.
Q Factor - Quality Factor notifies the patient to repeat the
test in case of a cough or any suspect blow.

Warnings
Note: Please read all the information in this manual
before using the
eSenseTM Piko®.
•The user should be under the care of a licensed
healthcare professional. Healthcare professionals may
recommend the
eSenseTM Piko®
as part of a
disease management treatment plan.
•Regardless of displayed test results, if signs or
symptoms of chest tightness, shodness of breath,
coughing or wheezing occur, contact your healthcare
professional immediately.
•The
eSenseTM Piko®
is intended for use by a single
person. Follow instructions carefully to get a correct
reading.
•If you use the
eSenseTM Piko®
above 1,000 feet
elevation (300m), adjust your values by adding 1.5%.
To do this, multiply the PEF & FEV1 by 1.015.
•The
eSenseTM Piko®
is not designed or intended to
be used as a primary diagnostic tool for asthma,
COPD, or any other pulmonary disease.
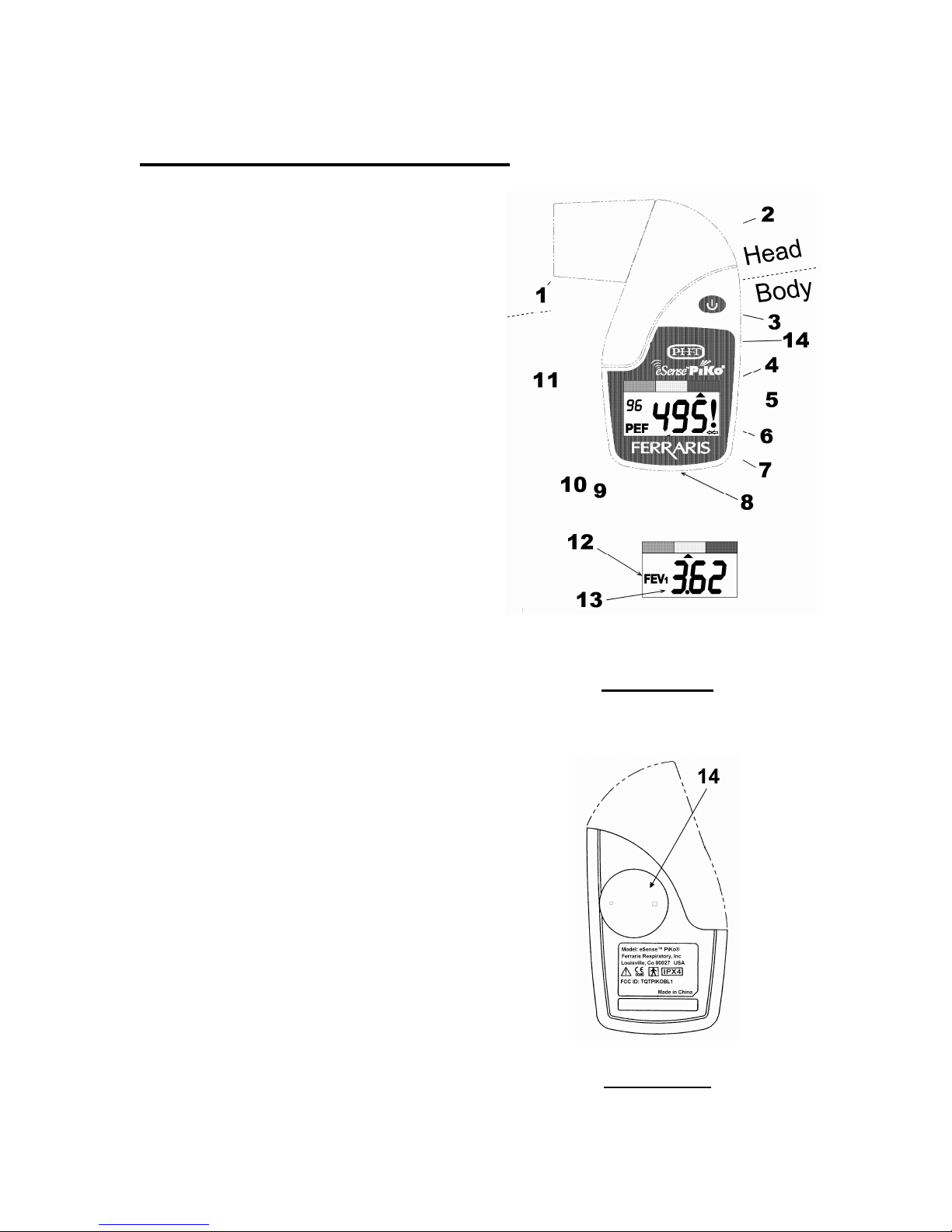
eSense
TM
Piko® Description
1. Removable Mouthpiece
2. Vent holes
3. Operate Button
4. Colored Zones
5. Zone indicator
6. Q-Factor indicator
7. Low battery indicator
8. IR Communication
window
9. PEF data, (Liter per
minute)
10. PEF indicator
11. Memory counter
12. FEV1 indicator
13. FEV1 data, (Liters)
14. Battery cover, (behind)
Note: The PEF data, items 9 &
10, and the FEV1 data, items
12 & 13, alternate at 2 second
intervals.
Note: The display turns blank if
the operate button is not
pressed for 3 minutes. Upon
pressing once the operate
button, the display will show the
last test results.
Front View
Back View
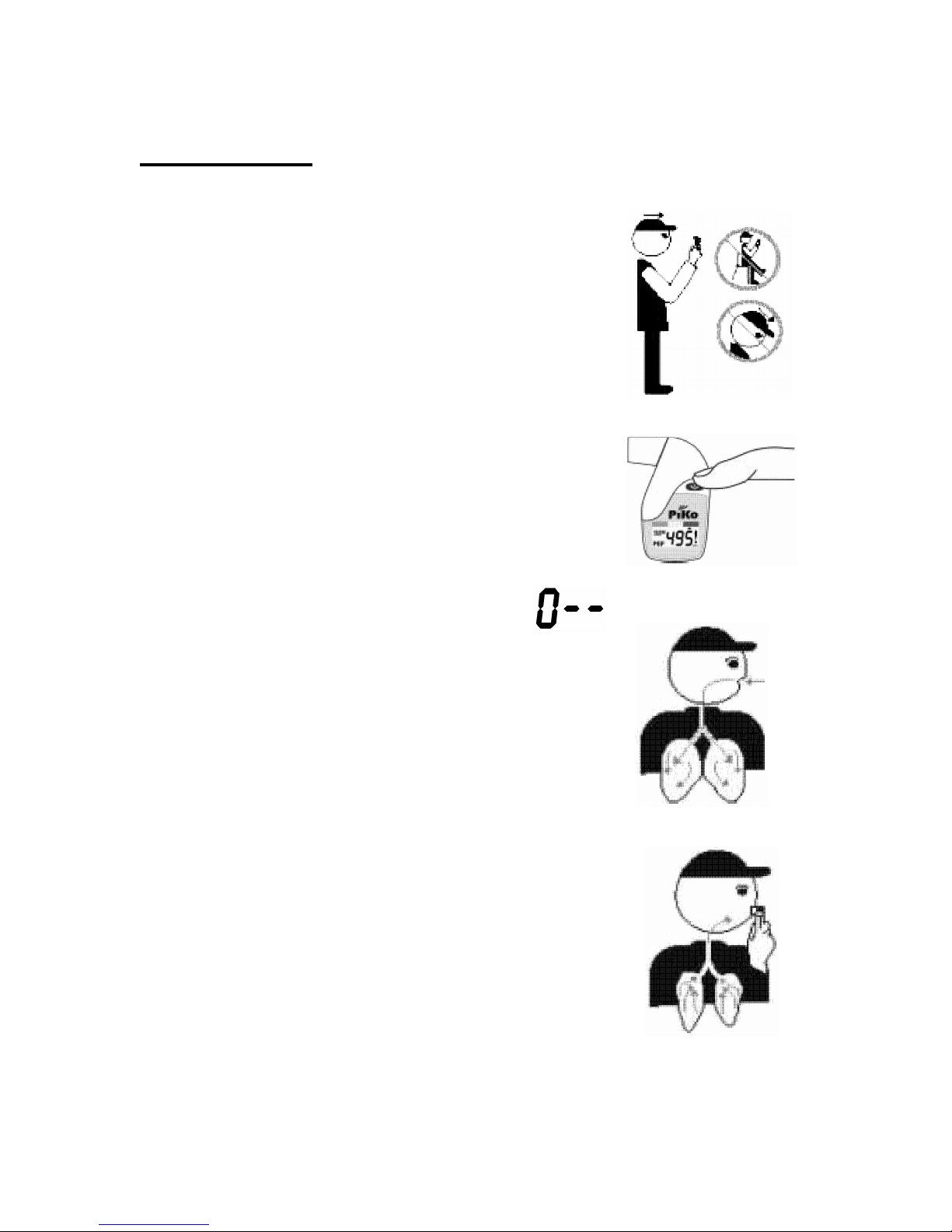
Perform Test
If the display is blank, press the operate button
once. The display will show the last test results.
1. Stand up. Hold the eSense
TM
Piko
®
in
your hand. Do not cover the vent holes.
2. Bring the eSense
TM
Piko
®
up with the
mouthpiece towards your mouth.
3. Press the operate button. A short beep
will confirm the action.
4. Wait to hear the second beep. The blow
animation will indicate that the test can
begin.
5. Inhale as fully as you can.
6. Place the mouthpiece in your mouth and
blow as hard as you can for several
seconds.
7. Your test results are displayed as soon as
you complete the test.
8. If a reference value was set, a zone indicator
will show your color zone.
9. You may want to perform 3 Tests.
10. The
eSenseTM Piko®
will select and save
only the best results for any tests performed
within 3 minutes.

Setting Reference Values
Setting a Reference PEF or FEV1 for color-zone display:
1. Press the Operate button quickly 4 times during 2 seconds. The
PEF or FEV1 indication starts blinking. The display will show
the stored reference value, (000 for first time setting).
2. Release the button, then each press will advance the display by
10 LPM for PEF, or 0.10 Liter for FEV1.
3. Hold the button for more than 5 seconds to save the desired
reference value. The PEF or FEV1 indicator will stop blinking
and a beep will sound.
4. The display will show briefly the new reference value and return
to show the last test results.
Notes:
•During the reference setting process, if the button is not pressed
within 5 seconds, the unit will exit the Setting Mode without
modifying the old reference. The display will return to the last test
results.
•The color-zone and relative warning indicator in the memory
review mode will reflect the new reference value.
•The entire memory bank can be erased by setting the PEF
reference to 10, or the FEV1 reference to 0.10.
•The optional accessory software can be used to toggle between
displaying the color-zone relative to the PEF or FEV1 reference.
•The optional accessory software can be used to modify each
color-zone limits, in 10% increments.

Memory Review
The eSense
TM
Piko
®
memory bank can store the last 96 PEF & FEV1
tests. Each memory location contains also the warning indicators
and a time/date stamp of the test.
To scroll through the memory bank, follow these steps:
1. Press and hold the Operate button for 5 seconds. The display
will show the last test results containing the PEF, FEV1, color-
zone and warning indicator.
It will also show the respective memory counter location.
2. Release the button. Each press of the button will now display
the next oldest stored test.
3. There are 2 ways to exit the Memory Review Mode:
•Press and hold down the Operate button for 5 seconds
•Avoid pressing the Operate button for 20 seconds.
The unit will then display the latest test result and will be ready
for a new test.
Note:The entire memory bank can be erased by setting the PEF
reference to 010, or the FEV1 reference to 0.10
Note:The time/date stamp of each test is accessible by the optional
accessory software for displaying trends and other information. It is
not displayed in the Memory Review Mode.
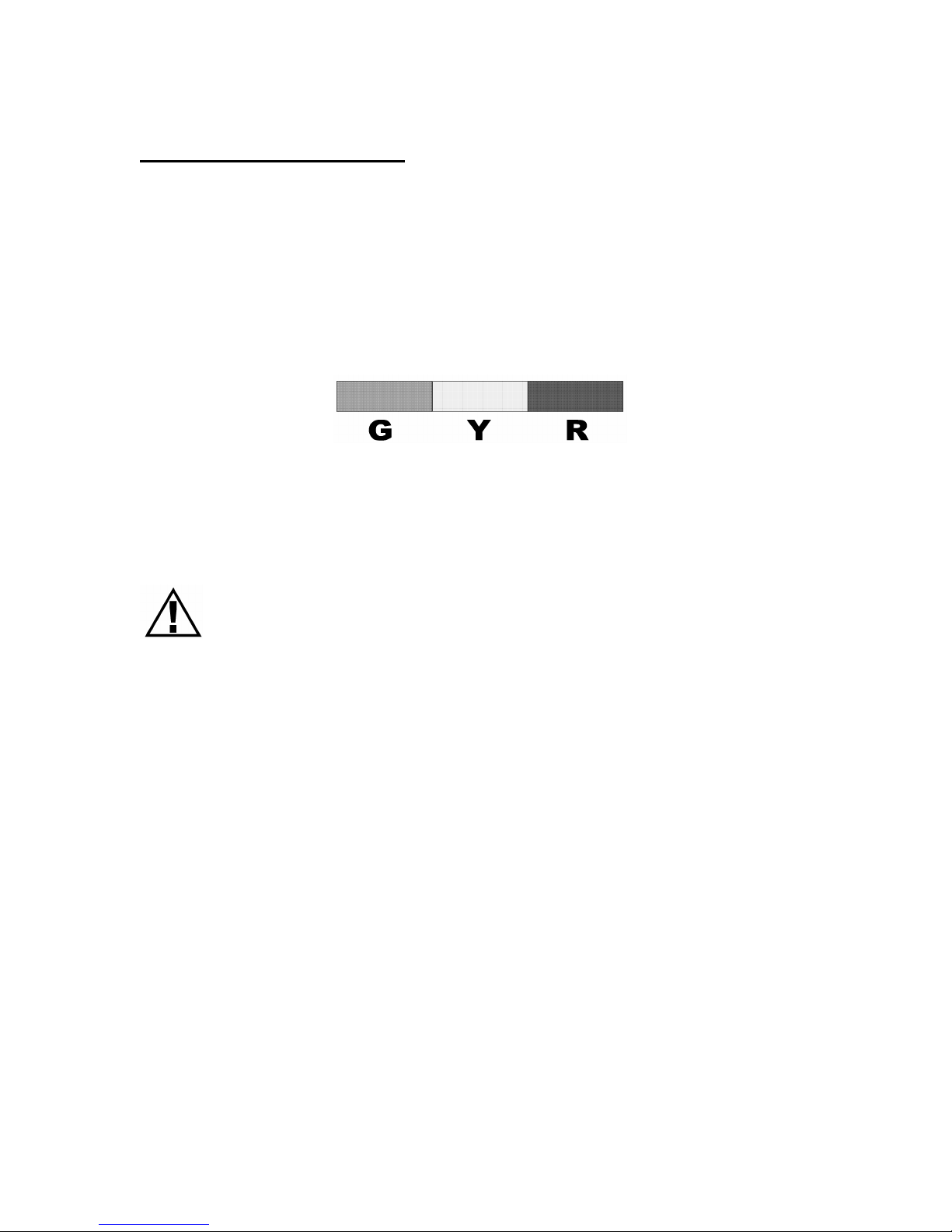
Additional Information
A reference PEF or FEV1 value is usually the best result you achieve
when you are not having symptoms.
Reference PEF or FEV1
To turn ON the color-zone indicator, you or a healthcare professional
must set a reference value (see page 6).
The following are color-zone descriptions:
Default Settings:
G Green = test results over 80% of the set value
Y Yellow = test results between 50 - 80% of the set value
R Red = test results under 50% of the set value
Note: If your test result falls in the Red Zone, contact your
healthcare professional immediately.
Q Factor Indicator
Indicates to repeat the test since one or more of the following
occurred:
•A cough was detected.
•The blow effort was too short.
•The blow effort had a slow start.
•The result was unnaturally low or high for your reference.
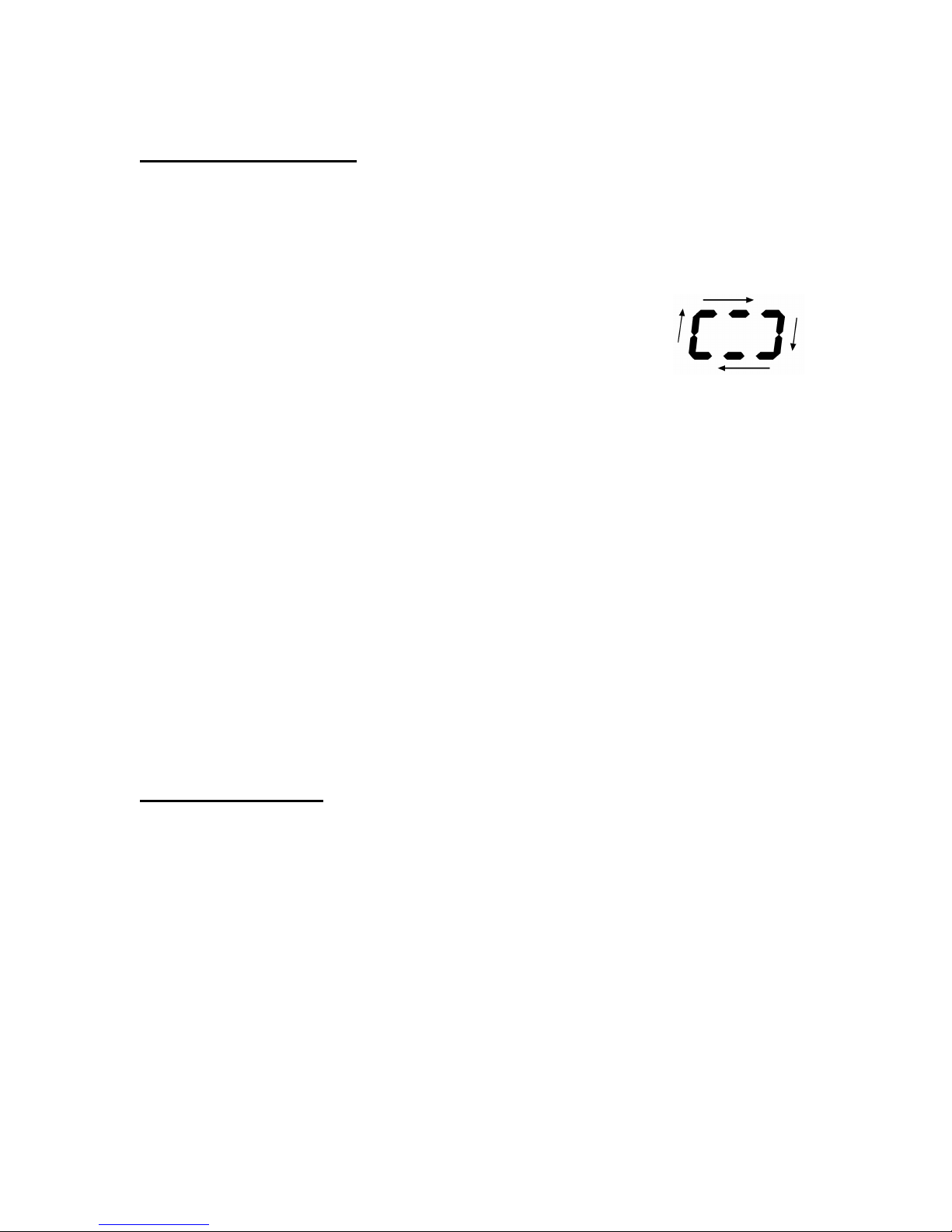
Downloading Data
The memory bank can be downloaded to a PC using an IR Cradle
and the PiKoNet software. The following is a description of the steps
involved:
1. To start downloading, press the Operate button.
The downloading progress is indicated by the
cycling segments on the display.
2. Upon completion of the downloading, the memory bank is
cleared and the display turns to show the latest test results.
3. The PiKoNet software shows trends and other relevant
information, (refer to the Manual of the optional program).
Note: It is possible to interrupt the downloading process at any time
by removing the eSense
TM
Piko
®
from the IR cradle.
The memory bank can also be downloaded to a LogPad (Palm PDA)
via Personal Area Network using the built-in transceiver. Please see
LogPad manual for instructions downloading eSense
TM
Piko
®
data.
Sound Patterns
•Pressing Operate Button: Short beep
•Error During Initialization: Long beep
•Blow Command: Short beep
•Result within Green Zone: No beep
•Result within Yellow Zone: 2 short beep
•Result within Red Zone: Long beep
•Any result containing (!): Long beep

Maintenance
Note: This product is NOT user serviceable.
Cleaning:
Note: Do not submerge the eSense
TM
Piko
®
in water.
1. Detach the mouthpiece by sliding it sideways.
2. Wash the mouthpiece with a liquid dish soap in cold water,
rinse and dry thoroughly. DO NOT use heat to dry.
3. The eSense
TM
Piko
®
can be cleaned as follows:
•Rinse with low-flow pressure water at room temperature.
•Only the Head Section may be cleaned with a solution of 5%
liquid dish soap in cold water, followed by rinsing.
•Hold the unit upside down and shake to remove the water.
•Wipe exterior with a clean, dry towel.
•Air dry completely before further use or storage.
4. To avoid damage to the unit:
•DO NOT immerse in water or use a dishwasher.
•DO NOT use a high pressure water flow.
•DO NOT use any sort of active solvent for cleaning.
•DO NOT dry by heat, hair dryer, or a dishwasher.
•DO NOT insert anything into the Vent Holes or the
Mouthpiece opening.
Battery Replacement:
1. Replace batteries when “Low Battery” indicator appears.
2. Open the battery door using the special tool supplied with the
eSense
TM
Piko
®
.
3. Use TWO, type 357 Silver Oxide button cell batteries, or
equivalent.
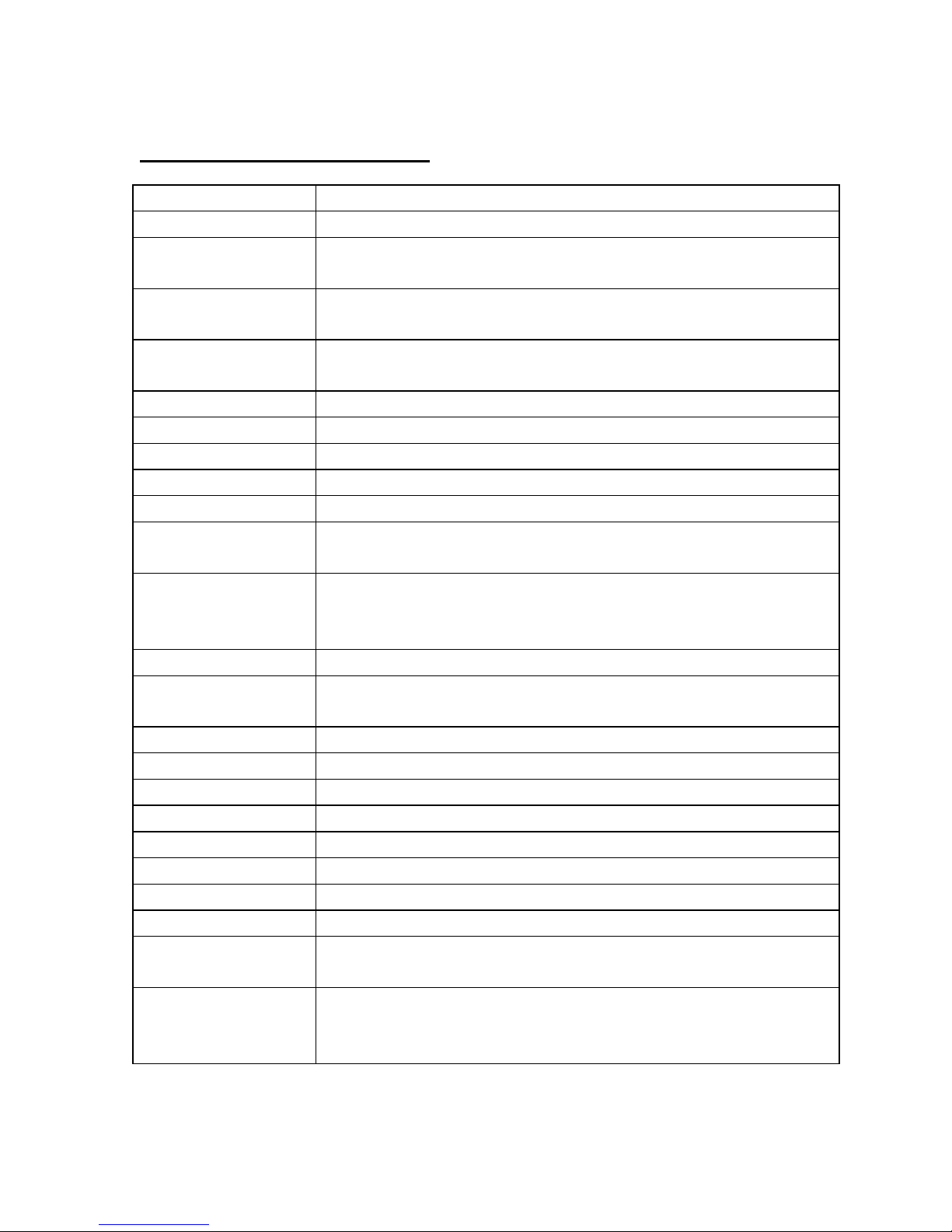
Technical Specifications
PEF Range 15 - 999 LPM, (1LPM resolution)
FEV1 Range 0.15 - 9.99 Liter, (0.01 Liter resolution)
Accuracy PEF: +/-5% or 10 LPM, (whichever is greater) FEV1
:
+/-3~5% or 0.05 Liter, (whichever is greater)
Sensor Pressure/flow sensor technology (US Patent
6,447,459)
Memory 96 Tests containing: PEF, , Color Zone, Quality
Factor, Time/Date Stamp (15 mm. resolution)
Memory type Nonvolatile
Color Zones 3 color Zones, (Green, Yellow, Red)
Reference values
User settable for PEF or FEV1
Quality Factor Warning & indicator for cough or abnormal blow
Sounds 4 patterns for different indications and warnings
Communication Bi-Directional IR port, (RS232 format)
RF via proprietary protocol
External settings Possible settings using the optional software:
- Each Color Zone limits, (in I 0% increments)
- Select Coior Zones to relate to PEF or FEV1
Battery 2, type 357 Silver Oxide button cells, (or equivalent)
Battery life 0.5 year (based on average of 3 Tests/day), or
approximately 5,400 consecutive Blows.
Dimensions 75 X 35 X 20 mm, (3 X 1.3 X 0.8 inch)
Weight 35 gm, (1 .25 oz)
Back pressure 1.5 cm H
2
O/LPS @ 14 LPS or lower
Operating Temp. 10 to 38 °C, 50 to 100 °F
Storage Temp. -20 to 60 °C, -4 to 140 °F
Humidity 0 - 100% relative humidity
Barometric 550 to 780 mmHg
Standard ATS1994, (monitoring devices)
Compliance AS/NZS-4237: I 994 EN60601, EN60601-1-1,
EN60601-1-2
Regulatory FDA - 510(k) for OTC
CE (0086) - Class I, with measurement function
FCC Part 15

Warranty
Ferraris Respiratory, lnc.(FRI) warrants each hardware product it
manufactures to be free from defects in material and workmanship.
Under this warranty, FRI will repair or replace at its option, any
hardware product or part thereof within 6 months of purchase.
Examination of the product must disclose to FRI that the product is
defective This warranty does not apply to any product or part that has
been repaired or altered in any way that, in FRI’s judgment, affects its
durability or reliability or any product or part subjected to misuse,
negligence or accident. THIS WARRANTY IS IN LIEU OF ALL
OTHER WARRANTIES OF MERCHANTABILITY AND FITNESS. A
copy of the receipt must accompany a warranty claim. If the product
should become defective within the warranty period, return to FRI at
901 Front Street, Louisville CO 80027 USA.
Safety
AS/NZS-4237:1994
EN60601
EN60601-1-1
EN60601-1-2
Type BF patient applied part.
Ordinary equipment (not protected against harmful ingress of
moisture).
Not suitable for use with flammable anesthetics.
Suitable for continuous operation.

This
symbol
indicates
that the
user must
read and understand
all instructions and
warnings.
This
symbol
indicates
that this
Class I
equipment with
measurement function
complies with the
European Union
Medical Device
Directive.
This
symbol
indicates
that this
device provides a
certain level of
protection against
electrical shock.
Ferraris Respiratory, Inc.
901 Front Street, Louisville, CO 80027, USA
Tel: 303-666-5555, Fax: 303-666-5588
Website: www.ferrarisrespiratory.com
In Europe: Hartforde Court, John Tate Road, Hertford,
SG13 &NW, UK
Table of contents
Popular Measuring Instrument manuals by other brands

Chauvin Arnoux
Chauvin Arnoux D38N manual

Precision Digital Corporation
Precision Digital Corporation vantageview PD6700-0L1 instruction manual

DMP Electronics
DMP Electronics DUALCOM Series Programming and installation guide

M-system
M-system 47DV instruction manual

VOLTCRAFT
VOLTCRAFT HygroCube 100 operating instructions

ABB
ABB COPA-XM 3000 Series instruction manual