6
6. Use ofECG3adjacenttoorstacked with otherequipmentshouldbe avoided
becauseit could result in improperoperation.If such use isnecessary,ECG3and the
otherequipmentshould be observed to verifythattheyareoperating normally.
7. Portable RFcommunicationsequipment(includingperipheralssuchasantenna
cablesand externalantennas)should beused no closerthan30 cm(12 inches)toany
partoftheECG3,includingcablesspecified bythemanufacturer.Otherwise,
degradationofthe performanceofthisequipmentcouldresult.
8. The useofaccessories,transmittersandcablesotherthanthose specifiedbyAndon
Health,with the exception ofaccessoriesand cablessoldbyAndonHealthof
PORTABLEECGMONITORasreplacementpartsforinternalcomponents,mayresult
inincreased EMISSIONSordecreased IMMUNITYofthePORTABLE ECGMONITOR.
9. FollowingESSENTIALPERFORMANCE isintendedused in Homecare environment.
ESSENTIALPERFORMANCE: Heartrate Accuracy: 2BPMor 5%(whichislarger)
10.The ECG3 can beusedathomeandin a communityhospital.IfuseECG3 inahospital,
keepthe unitawayfromactiveHFSURGICAL EQUIPMENTandthe RFshieldedroom
ofan MESYSTEMformagneticresonanceimaging,where theintensityofEM
DISTURBANCES ishigh.If useECG3athome,keeptheunit awayfromdevices
emitting strongelectromagneticradiosuchasmobile phone and microwave.
11.Uploadingand watching theECGdatarecorded in theECG3inaniOS deviceisthe
onlyoperation thatusercan doinanambientcondition with highintensityofEM
DISTURBANCES.
12. Donotusethisproductwithadefibrillator,whichwillbe dangerous.Removethe
ECG3 before usingadefibrillator.
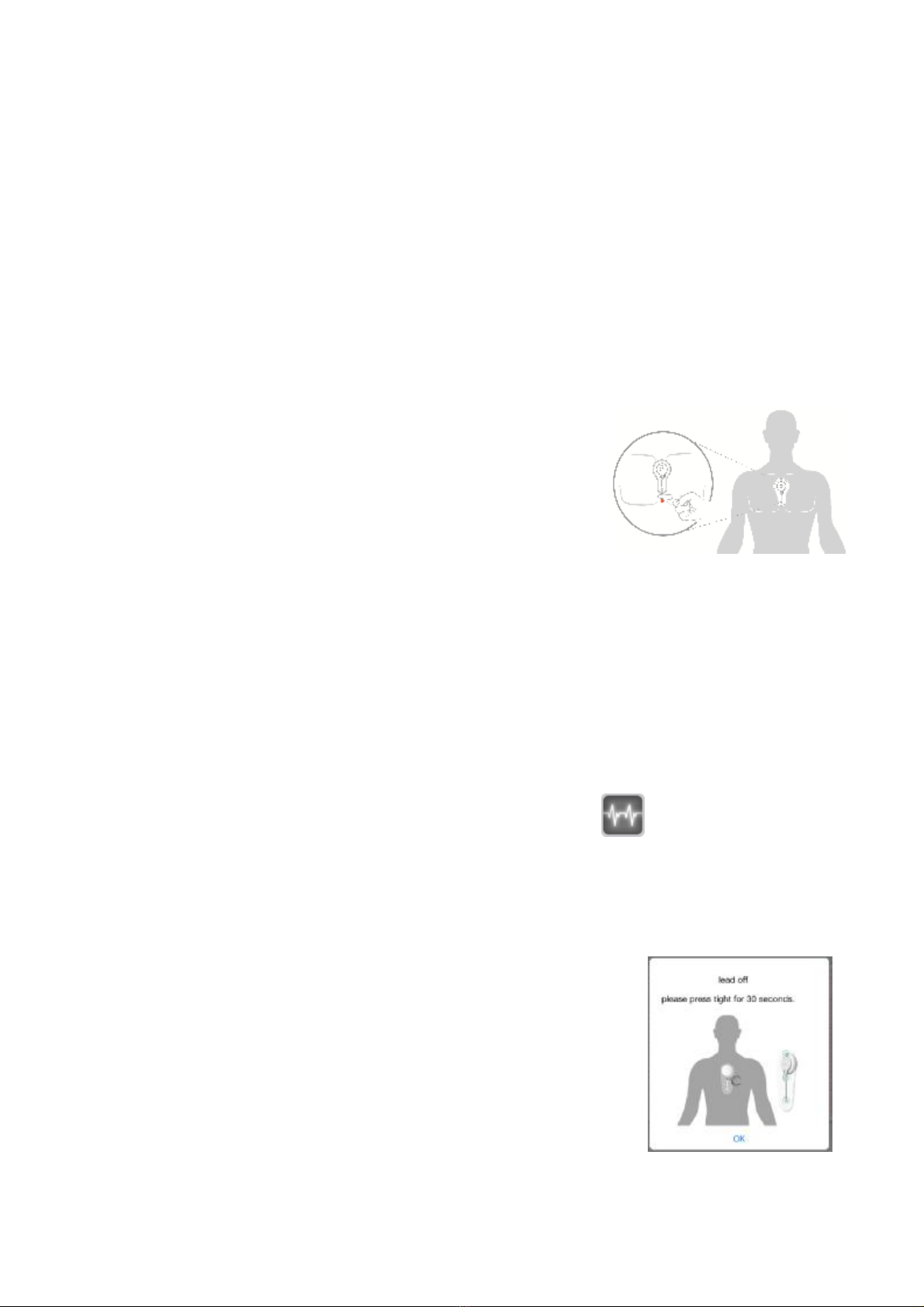
13.The skin which theelectrodesare contactedorpastedshouldbe clean,because
excessivebodyhairwillinfluencethe ECGmeasurement.
14. If a skinallergyorotherunfitted symptomshappenedduringmeasurement, stop
measuringimmediately.
15. Please donotshare the unitwithanyinfectiouspersontoavoidcross-infection.
16.Sweatandsteamhavenoharmfuleffecton ECG3,butyoushouldkeepthisunitdryas
possible.Avoidactivitiesinwetenvironmentsuchastaking ashower.
17.The ECG3 willnotsend warning message touserwhena heartabnormalityoccurs
duringtakingmeasurement.
18. Wrong operationandinappropriatepositionofelectrode will lead toanunreliable
measuringresult. Please read the Owner sManualcarefullyandoperatetheECG3
cautiouslywhiletaking measurement.
19.Avoidstrenuousexercise while taking measurement,whichmaycause awrong
measuringresult.
20.The devicewouldnotapplytothepatientswho useanartificialheartandlung(there
will be no ECGwaveform).
21.The patientcanbeanintendedoperator.Allfunctionsoftheunitcanbeusedsafelyby
patient.Chargingisthe onlymaintenance can be operatedbypatient.
22.Pleasedon tuseaccessories(e.g.electrode,datacable,etc.)thatarenotdescribedin
the instructions.Dothe opposite willpossiblydamage thisunitand causedangers.
23.Thisproductshouldnotbeused asa USBdevice.
24. Thisproductmightnotmeetitsperformancespecificationsif stored orused outside
the specified temperature and humidityranges.
25. No maintenancecanbe carried onwhentheECG3isworking ona patient.