SIGHT SCIENCES TearCare User manual

Document Number 07418 Rev C
January 2022
© 2022 Sight Sciences. All rights reserved.

Instructions For Use

TEARCARE.COM

SmartHub INSTRUCTIONS FOR USE
1 INTRODUCTION........................................................ 1
1.1 Contacting Sight Sciences ..................................... 1
2 TEARCARE SYSTEM INFORMATION .................... 1
2.1 Indications for Use................................................... 1
2.2 Contraindications ..................................................... 1
2.3 Warnings .................................................................... 1
2.4 Precautions................................................................2
2.5 Potential Adverse Effects of the Device
on Health....................................................................2
2.6 For Prescription Use only.......................................3
2.7 Clinical Study Summary..........................................3
2.8 Device Description................................................. 12
2.8.1 TearCare SmartHub................................................ 12
2.8.2 TearCare SmartLids................................................ 14
3 PERFORMING A TEARCARE PROCEDURE....... 15
3.1 Unpacking and Checking the
TearCare System Products ................................... 15
3.2 Preparing the Patient ............................................ 15
3.3 Applying the SmartLid ......................................... 16
3.4 Plugging the SmartLids into the SmartHub ..... 17
3.5 Starting a TearCare Procedure ........................... 18
3.6 Adjusting temperature ......................................... 19
3.7 Terminating a procedure early ........................... 20
3.8 Expressing the meibomian glands..................... 20
4 PRODUCT DISPOSAL ........................................... 20
5 CHARGING THE SMARTHUB................................ 21
6 USER MAINTENANCE........................................... 22
6.1 Cleaning the SmartHub........................................ 22
7 TROUBLESHOOTING ............................................ 23
7.1 SmartHub Troubleshooting ................................. 23
7.2 SmartLid Troubleshooting................................... 26
8 SPECIFICATIONS....................................................27
8.1 Thermal Output ......................................................27
8.2 Physical Components ............................................27
8.3 Environmental Conditions ....................................27
8.4 Standards Compliance ......................................... 28
8.4.1 Electromagnetic Emissions ................................. 29
8.4.2 Essential Performance.......................................... 30
8.4.3 EMC Susceptibility ................................................ 30
APPENDIX A: Symbols..................................................... 31
APPENDIX B ...................................................................... 34
Product Returns..................................................... 34
Responsibility of Sight Sciences........................ 34
Adverse Event Reporting .................................... 34
Manufacturer .......................................................... 34
Patents and Trademarks ...................................... 34
Table of Contents

1
1.1 CONTACTING SIGHT SCIENCES
All questions or concerns about the TearCare® System products
should be directed to:
Sight Sciences, Inc.
4040 Campbell Ave.
Suite 100
Menlo Park, CA 94025
Telephone: (877) 266-1144
Website: www.sightsciences.com
2. TEARCARE SYSTEM INFORMATION
2.1 INDICATIONS FOR USE
The TearCare® System is intended for the application of localized
heat therapy in adult patients with evaporative dry eye disease
due to meibomian gland dysfunction (MGD), when used in
conjunction with manual expression of the meibomian glands.
2.2 CONTRAINDICATIONS
TearCare is contraindicated for patients with the following
conditions. Use of the device in patients with these conditions
may cause serious injury or exacerbation of the condition.
• Recent (i.e. within the last 90 days) surgical procedure to the
eye or eyelid.
• Recent ocular injury.
• History of Herpes Simplex or Herpes Zoster of the eye
oreyelid.
• Active ocular or periocular infection, inflammation
orirritation.
• Diminished or abnormal facial, periocular, ocular, or corneal
sensation.
• Ocular surface ulcers.
• Hordeolum, stye, or chalazion.
• Do not use TearCare in patients under the age of 22 years.
• Pacemakers or implantable cardiac defibrillators (ICD).
Useof the TearCare System may aect the performance
ofpacemakers or ICD’s due to electromagnetic interference
(EMI). To avoid any potentially serious safety eects,
patientswith these implants should not be treated with
theTearCareSystem.
• Known allergy to acrylate.
• Known allergy to silicone tissue adhesives.
• Known allergy to copper.
2.3 WARNINGS
• Do not use the TearCare System outside the instructions
for use described in this manual. Doing so can result
inunanticipated patient harm.
• Do not attempt to connect the SmartHub or SmartLid
directly to an electrical outlet of any kind.
• Do not use the TearCare System in or near an MRI suite
ornear a magnetic field. Serious injury can occur to a patient
or technician if a TearCare system is brought into an MRI suite.
• The TearCare System has not been tested in the presence
of flammable anesthetics or other flammable agents in
combination with air, nitrous oxide, or oxygen enriched
environments.
• Do not apply SmartLids to non-intact skin (i.e., skin with
active abrasion, cut, burn, rash, inflammation, redness, etc.)
1. INTRODUCTION

2
2.4 PRECAUTIONS
• Use of the TearCare System in patients with eyelid
abnormalities (e.g., entropion, ectropion, tumor, edema,
blepharospasm, lagophthalmos, severe trichiasis, severe ptosis,
etc.) may result in poor adhesion of the SmartLid to the eyelid
and/or reduced benefit.
• Use caution when using the TearCare System in patients with
ocular surface abnormalities (e.g. pterygium, pingueculum,
corneal dystrophies, etc.) as the heat delivered by TearCare
may aggravate these conditions.
• Remove contact lenses from the patient’s eyes prior to use of
TearCare. Patients should wait 60 minutes after the completion
of the TearCare procedure before re-inserting contact lenses.
• Do not apply the SmartLids to any other part of the patient’s
body including the cornea. The SmartLids are only intended
forapplication on the external surface of the patient’s eyelids.
• It is important for patient to keep their eyes open (blinking is
permitted) during treatment, to allow heat to dissipate o of
the ocular surface.
• Eectiveness of the TearCare System has not been established
in subjects for whom the treatment temperature is lowered
from Warmth Level Setting #5 due to patient pain or
discomfort.
• Do not reuse the SmartLids. Cross-contamination can occur
ifre-use is attempted.
• Do not use the TearCare System, its components, or accessories
that appear damaged. Inspect all components for damage
before each use.
• The safety and eectiveness of the TearCare System is not
known in the following patient populations that were excluded
in the OLYMPIA pivotal study: patients under 22 years of age,
dry eye signs and symptoms other than meibomian gland
dysfunction, severe signs and symptoms of dry eye due to
meibomian gland dysfunction (OSDI > 79), mild signs and
symptoms of dry eye due to meibomian gland dysfunction,
andother study exclusions described in Section 2.7 “Clinical
Study Summary.”
2.5 POTENTIAL ADVERSE EFFECTS OF THE DEVICE
ONHEALTH
Below is a list of potential adverse eects that may be associated
with use of the TearCare System. Adverse eects that occurred
in the OLYMPIA pivotal clinical trial are indicated with an asterisk
(*) and additional information regarding these adverse eects is
summarized in Section 2.7 “Clinical Study Summary.”
Potential adverse eects may include but are not limited to:
• Eyelid or eye pain requiring discontinuation of the treatment
procedure
• Eyelid irritation or inflammation*
• Ocular surface irritation or inflammation*
• Ocular symptoms (such as burning, redness, tearing, visual
disturbance, redness)
• Burning, erythema, or swelling of the eyelids
• Conjunctival infection (moderate or severe)
• Conjunctival abrasion
• Corneal abrasion
• Corneal deformation
• Allergic or inflammatory reaction to medical adhesive on the
SmartLid device
• Formation of a chalazion or stye*
2. TEARCARE SYSTEM INFORMATION

3
• Decline in visual acuity*
• Worsening of dry eye symptoms*
• Increased discomfort or pain of ocular surface (grittiness,
foreign body sensation, etc.)
• Discomfort or pain of eyelids or orbit*
There is a potential risk of thermal injury to eye or eyelid based
onthe device design.
2.6 FOR PRESCRIPTION USE ONLY
Federal (USA) law restricts this device to sale, distribution, or use by
or on the order of a physician. Physician training is required prior to
use of the TearCare System.
2.7 CLINICAL STUDY SUMMARY
A prospective, multicenter, randomized, non-inferiority, masked,
controlled clinical trial (“OLYMPIA”) was performed to demonstrate
the safety and eectiveness of a single TearCare System treatment
compared to a single LipiFlow Thermal Pulsation System to treat
adult patients with Meibomian Gland Dysfunction (MGD).
Study Design
This study was a prospective, randomized, single-masked,
multi-center, non-inferiority, non-significant risk device study.
Randomized subjects were followed for one month succeeding
treatment with follow-up data collected at Day 1, Week 2, and
1 Month. A total of 235 subjects (470 eyes) from 10 investigative
centers in the United States participated in the study, comprised
of 169 female and 66 males, ages 22 to 91 years (mean = 55.9 ±
14.4 years). Subjects were randomized 1:1 to receive either a single
TearCare System or LipiFlow System treatment. The TearCare
treatment arm consisted of a 15-minute thermal procedure
followed immediately (i.e., within 3 minutes) by manual expression
of the meibomian glands using the Clearance Assistant. Study
subjects were grouped into two cohorts to account for a SmartLid
design change made during the study. There were 93 subjects
in Cohort 1, comprised of 47 LipiFlow and 46 TearCare subjects
treated with the prior SmartLid design. There were 142 subjects
in Cohort 2, comprised of 73 LipiFlow and 69 TearCare subjects
treated with the current SmartLid design. The eectiveness
endpoints were assessed using data from Cohort 2 and the safety
endpoints were evaluated separately for Cohort 1 and 2. The study
procedures took place between March 2019 and February 2020.
Study Endpoints
The primary eectiveness endpoints included the mean change
from baseline to 1-month in Tear Break-Up Time (TBUT) and
Total Meibomian Gland Secretion Score (MGSS). Secondary
eectiveness endpoints included the mean change from baseline
to 1-month in Ocular Surface Disease Index (OSDI) score, corneal
and conjunctival staining scores, Symptom Assessment in Dry Eye
(SANDE) scores, Eye Dryness Score and meibomian gland health.
The primary safety endpoint was the incidence of ocular adverse
events. The secondary safety endpoints included discomfort/pain
during and after the procedure, change in Best Corrected Visual
Acuity (BCVA), and change in intraocular pressure (IOP).
Description of Study Patients
To participate in the study, subjects were required to be at least
22 years of age with symptoms of dry eye in the past 3 months,
regularly reported use of artificial tears or lubricants over the
2. TEARCARE SYSTEM INFORMATION

4
past month to relieve dry eye symptoms, a Tear Break-up Time
(TBUT) of ≤7 seconds in both eyes, an OSDI score of 23-79,
and meibomian gland obstruction in both eyes based on a total
Meibomian Gland Score ≤12 in each eye with at least 15 glands
in each lower eyelid expressible with a sterile cotton swab at the
Baseline visit.
Subjects could not participate in the study if they were using dry
eye medications (such as lifitegrast, cyclosporine, antihistamines),
or systemic medications (such as diuretics, anti-hypertensives)
known to cause ocular dryness within specific timeframes prior
to enrollment, prior dry eye treatments (such as laser, thermal
pulsation, lid debridement, punctal plugs) within specific
timeframes prior to enrollment, history of eyelid, conjunctiva or
corneal surgery within the past year, use of bimatoprost, Retin
A or isotretinoin, systemic diseases resulting in dry eye (such
as Sjogren’s syndrome, lupus, Grave’s disease). Other exclusion
criteria included history of ocular Herpes Simplex or Herpes Zoster,
any active and clinically significant ocular or peri-ocular infection
or inflammation or anterior blepharitis or eyelid abnormalities
(such as entropion/ectropion, lagophthalmos) or dermatologic
or cutaneous disease of the eyelid or periocular area or ocular
surface abnormalities that may aect tear film distribution or
treatment (such as pterygium, anterior membrane dystrophy) or
conjunctivitis (such as allergic, vernal or giant papillary). Subjects
were also excluded if they had corneal surface abnormalities (such
as corneal epithelial defects, ulcers, dystrophies, keratoconus,
ectatic disease), recurrent clinically significant eye inflammation
(other than dry eye) or ocular trauma within 3 months prior to
enrollment. Based on the clinical judgement of the investigator,
subjects with meibomian glands having significant capping,
atrophy or unable to be expressed were also excluded from
thestudy.
Demographics
The mean age of all 235 enrolled and treated subjects was
55.9 ± 14.4 years. The gender of all randomized subjects was
similar across treatment groups with a combined distribution of
169 (72%) female and 66 (28%) male subjects. The demographic
of all study subjects was similar between the treatment groups.
Asummary of patient demographics is presented in Table 1.
Table 1. Baseline Demographics (Cohort 1 + Cohort 2)
TearCare
(n=115 subjects)
Lipiflow
(n=120 subjects)
Combined
(n=235 Subjects)
Age (years)
n 115 120 235
Mean (SD) 57 (14.0) 55 (14.5) 55.9 (14.4)
Median 60 56.5 58.0
Range (min-max) 22 – 91 23 – 86 22 – 91
Gender n(%)
Female 86/115 (74.8%) 83/120 (69.2%) 169/235 (71.9%)
Male 29/115 (25.2%) 37/120 (30.8%) 66/235 (28.1%)
Race, n(%)
American Indian/
Alaska Native
2/115 (1.7%) 0/120 (0.0%) 2/235 (0.9%)
Asian 3/115 (2.6%) 5/120 (4.2%) 8/235 (3.4%)
Black or African
American
4/115 (3.5%) 5/120 (4.2%) 9/235 (3.8%)

5
TearCare
(n=115 subjects)
Lipiflow
(n=120 subjects)
Combined
(n=235 Subjects)
Indian 0/115 (0.0%) 0/120 (0.0%) 0/235 (0.0%)
Iranian 1/115 (0.9%) 0/120 (0.0%) 1/235 (0.4%)
Middle Eastern 0/115 (0.0%) 1/120 (0.8%) 1/235 (0.4%)
Spanish 1/115 (0.9%) 0/120 (0.0%) 1/235 (0.4%)
White 104/115 (90.4%) 109/120 (90.8%) 213/235 (90.2%)
Ethnicity, n(%)
Non-Hispanic
or Latino
94 (82.1%) 101/120 (84.2%) 195/235 (83.1%)
Hispanic or Latino 21 (18.3%) 19/120 (15.8%) 40/235 (17.0%)
Eectiveness Results
The primary eectiveness endpoints were defined as the
change from baseline to 1 month for Tear Break-up Time (TBUT)
and total Meibomian Gland Secretion Score (MGSS) for both
treatment groups in Cohort 2. Subjects in both treatment groups
demonstrated a statistically significant improvement in both
endpoints and the TearCare arm of the study established
non-inferiority relative to the LipiFlow arm for both TBUT
andMGSS. Results are listed in Tables 2 and 3 below.
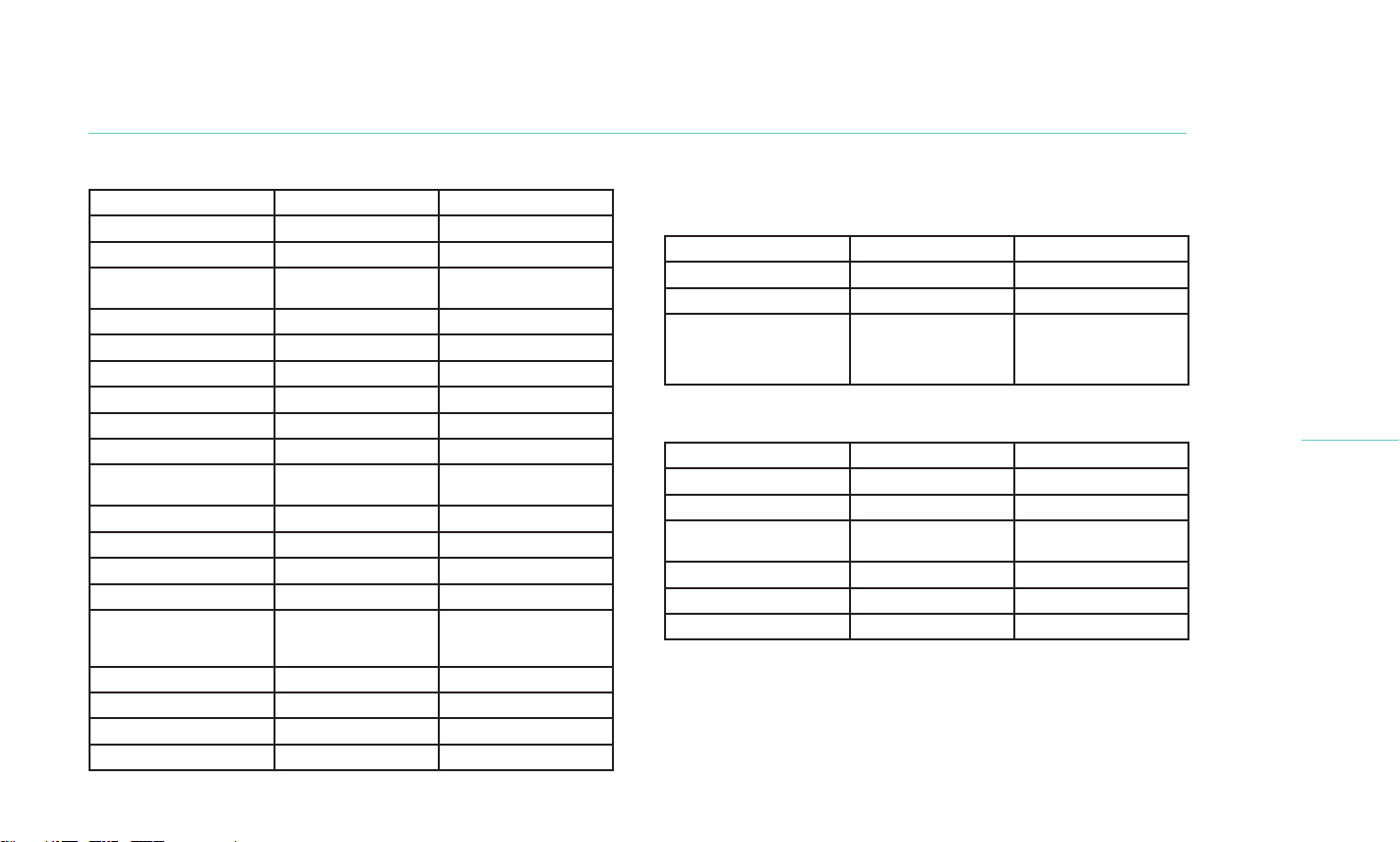
Table 2. Change in TBUT at 1 month compared to baseline
(Cohort 2)
Visit Parameter Statistics TearCare LipiFlow
Baseline
N (eyes) 134 136
TBUT
mean(sd) 4.62 (1.19) 4.49 (1.05)
median 4.65 4.36
min, max [1.12, 6.85] [0.87, 6.92]
95% CI [4.41, 4.82] [4.32, 4.67]
Month 1
N (eyes) 134 136
TBUT
mean(sd) 7.64 (4.64) 7.08 (3.19)
median 6.59 6.39
min, max [2.81, 32.50] [2.94, 23.83]
95% CI [6.84, 8.43] [6.54, 7.62]
TBUT change-from-
baseline
mean(sd) 3.02 (4.41) 2.58 (3.28)
median 1.95 1.90
min, max [-2.39, 28.00] [-1.75, 20.79]
95% CI [2.27, 3.78] [2.03, 3.14]
2. TEARCARE SYSTEM INFORMATION
6
Table 3. Change in MGSS at 1 month compared to baseline (Cohort 2)
Visit Parameter Statistics TearCare LipiFlow
Baseline
N (eyes) 134 136
Total Meibomian Gland
Secretion Score
mean(sd) 6.54 (3.11) 6.29 (2.75)
median 7.00 6.00
min, max [0.00, 12.00] [0.00, 12.00]
95% CI [6.01, 7.07] [5.83, 6.76]
Month 1
N (eyes) 134 136
Total Meibomian Gland
Secretion Score
mean(sd) 17.74 (11.63) 17.38 (11.08)
median 16.00 16.00
min, max [0.00, 45.00] [0.00, 41.00]
95% CI [15.75, 19.73] [15.50, 19.26]
Total Meibomian Gland
Secretion Score change-
from-baseline
mean(sd) 11.20 (11.13) 11.09 (10.41)
median 8.00 8.00
min, max [-8.00, 45.00] [-8.00, 36.00]
95% CI [9.30, 13.10] [9.32, 12.85]
Results of the secondary endpoints for both treatment groups
inCohort 2 are presented in the following tables:
Table 4. OSDI – Mean change from baseline at 1-Month (Cohort 2)
Parameter TearCare LipiFlow
N (subjects) 67 68
Baseline (sd) 52.0 ± 14.4 51.1 ± 16.1
1-Month
change (sd)
min, max
24.2 ± 17.7
-27.88 ± 20.5
-66.7, 35.0
27.7 ± 19.6
-23.4 ± 17.7
-59.1, 30.2
Table 5. Change in Total Corneal Staining at 1 month compared
tobaseline (Cohort 2)
Visit Parameter Statistics TearCare LipiFlow
Baseline
N (eyes) 134 136
Total Corneal
StainingScore
mean(sd) 2.51 (2.09) 2.51 (2.31)
median 2.00 2.00
min, max [0.00, 9.00] [0.00, 9.00]

7
Visit Parameter Statistics TearCare LipiFlow
Month 1
N (eyes) 134 136
Total Corneal
StainingScore
mean(sd) 2.25 (2.19) 1.93 (2.16)
median 2.00 1.00
min, max [0.00, 10.00] [0.00, 9.00]
Total Corneal Staining
Score change-from-
baseline
mean(sd) -0.25 (1.98) -0.57 (2.01)
median 0.00 0.00
min, max [-7.00, 7.00] [-7.00, 5.00]
Table 6. Change in Total Conjunctival Staining at 1 month
compared to baseline (Cohort 2)
Visit Parameter Statistics TearCare LipiFlow
Baseline
N (eyes) 134 136
Total Conjunctival
Staining Score
mean(sd) 4.08 (3.32) 4.85 (3.10)
median 3.00 4.00
min, max [0.00, 18.00] [0.00, 16.00]
Visit Parameter Statistics TearCare LipiFlow
Month 1
N (eyes) 134 136
Total Conjunctival
Staining Score
mean(sd) 3.43 (2.75) 4.07 (3.96)
median 3.00 3.00
min, max [0.00, 18.00] [0.00, 18.00]
Total Conjunctival
Staining Score change-
from-baseline
mean(sd) -0.66 (2.26) -0.78 (3.18)
median -1.00 -1.00
min, max [-7.00, 7.00] [-8.00, 14.00]
Safety Results
The primary safety endpoint was defined as the incidence of
ocularadverse events (AEs) and they are listed below in Table 7.
Nosubject in either group experienced any serious adverse events
or serious device related adverse events that required further
management. There were 4 device related AEs in the TearCare
group reported in 3 subjects (Chalazion-1, Superficial Punctate
Keratitis-2, Blepharitis-1) and 7 device related AEs in the LipiFlow
group reported in 4 subjects (Blepharitis-2, Foreign Body
Sensation-3, Dry Eye Disease-2). In the TearCare group, the subject
with chalazion required medication to resolve the event. In the
LipiFlow group, one subject was prescribed warm compresses for
blepharitis and another subject received medication to resolve
2. TEARCARE SYSTEM INFORMATION
8
aforeign body sensation. The observed rate of device related AEs
was 2.1% (n=2 AEs/92 eyes) and 2.1% (n=3 AEs/138 eyes)
respectively in Cohort 1 and Cohort 2 of the TearCare group and
1.0% (n=1 AEs/94 eyes) and 2.1% (n=3 AEs/146 eyes) respectively
in Cohort 1 and Cohort 2 of the LipiFlow group. There were 2.1%
(n=1 subjects/46) of subjects in Cohort 1 and 4.3% (n=3
subjects/69) of subjects in Cohort 2 experiencing one or more
device-related adverse events of the TearCare group and there
were 2.1% (n=1 subjects/47) of subjects in Cohort 1 and 4.1%
(n=3 subjects/73) of subjects in Cohort 2 of the LipiFlow group.
The observed rate of ocular AEs of any type was 4.3% (4 eyes/
92 eyes) and 3.0% (4 eyes/138 eyes) respectively in Cohort 1 and
Cohort 2 of the TearCare group and 3.2% (3 eyes/94 eyes) and
3.4% (5 eyes/146 eyes) respectively in Cohort 1 and Cohort 2 of
the LipiFlow group.
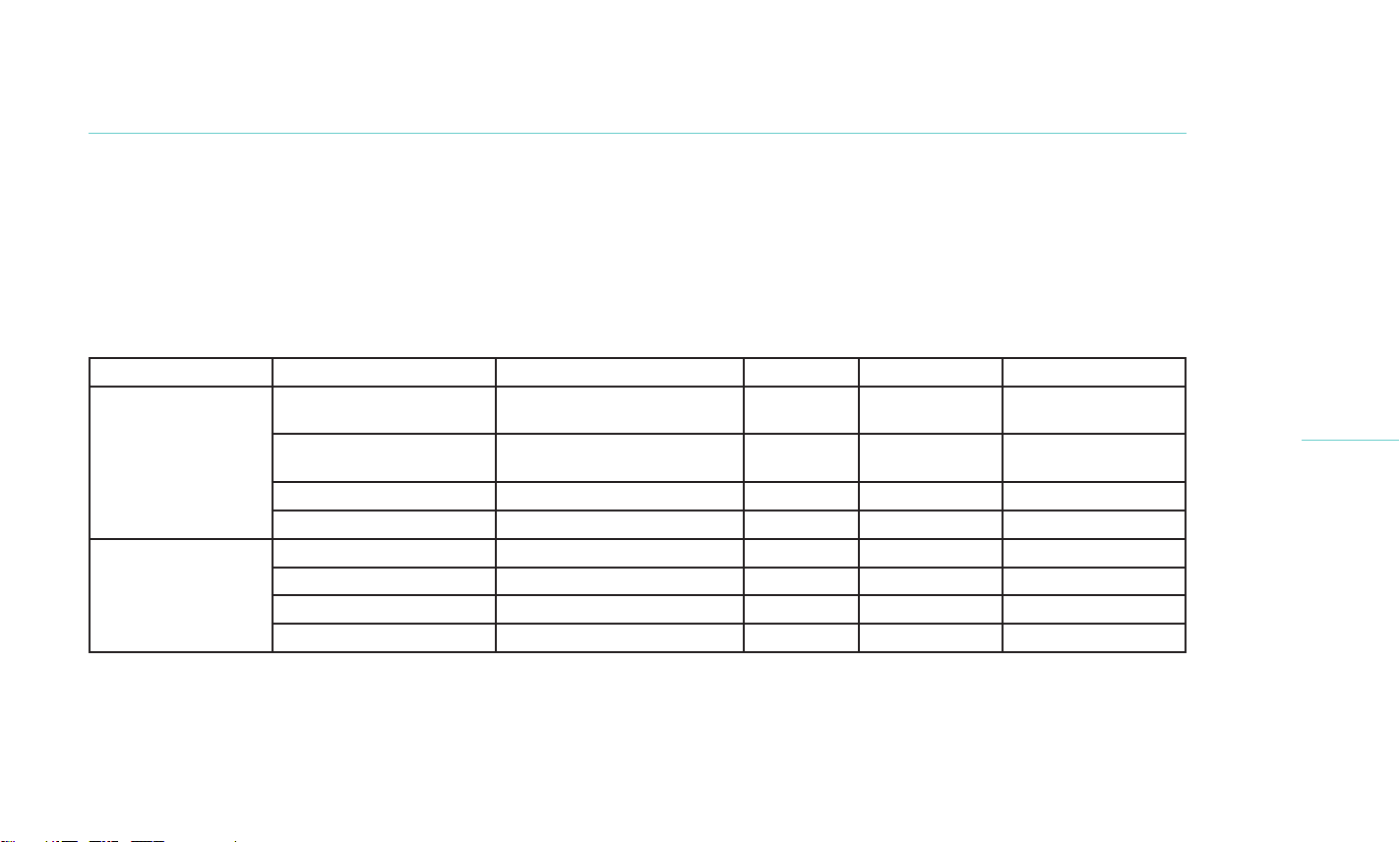
Table 7. Detailed list of ocular adverse events by treatment arm
Treatment Group AE description Relationship to Treatment Serious Action Taken AE outcome
TearCare
(Cohort 1)
Superficial Punctate
Keratitis (SPK)
Probably Related No None Resolved
Superficial Punctate
Keratitis (SPK)
Probably Related No None Resolved
Decrease in BCVA Unlikely Related No None Resolved
Conjunctival Injection Unlikely Related No None Ongoing
TearCare
(Cohort 2)
Chalazion Definitely Related No Medication Resolved
Blepharitis Possibly Related No None Resolved
Decrease in BCVA Unlikely Related No None Resolved
Iritis Definitely Unrelated No Medication Resolved
9
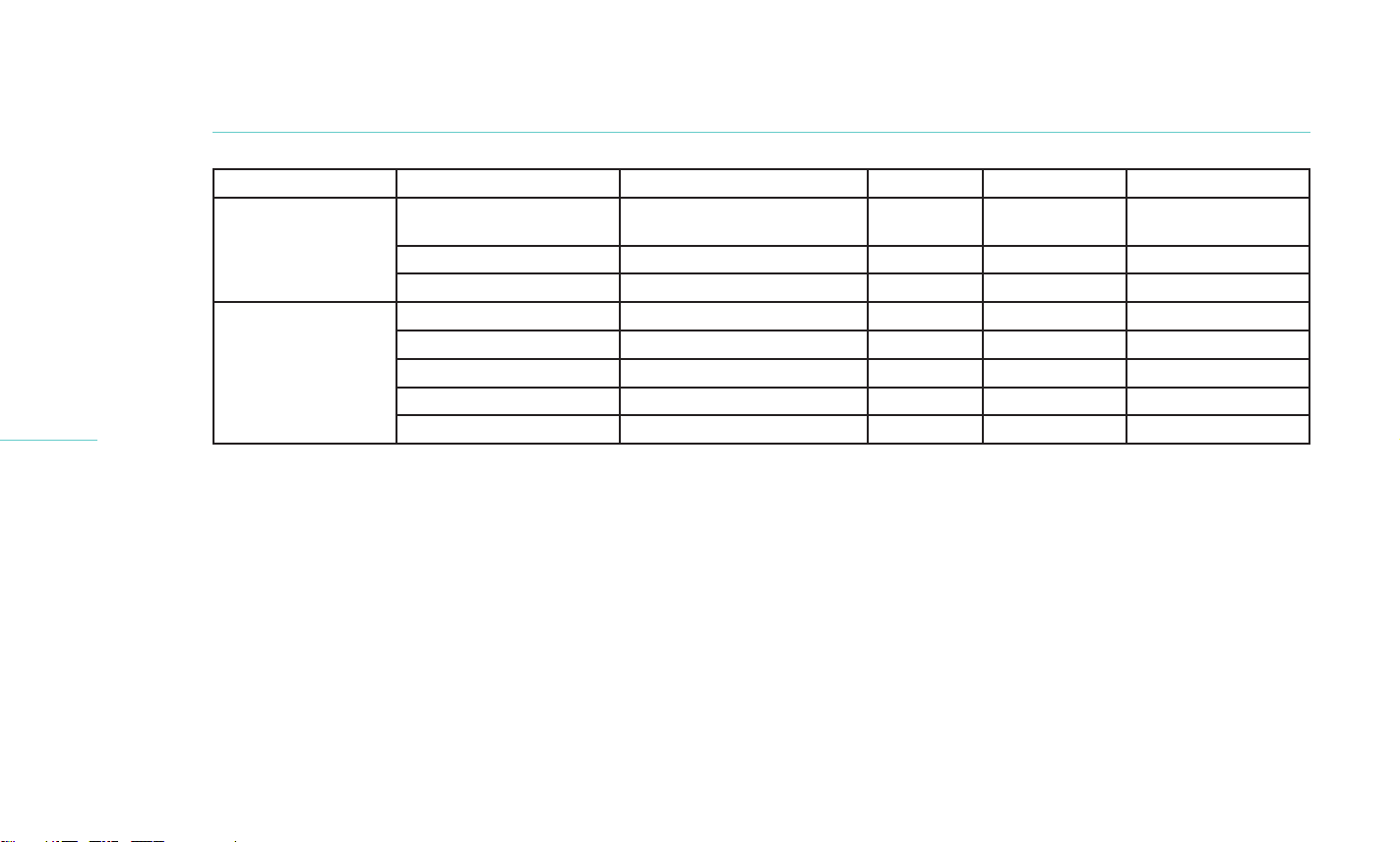
Treatment Group AE description Relationship to Treatment Serious Action Taken AE outcome
LipiFlow
(Cohort 1)
Blepharitis Possibly Related No Warm
compresses
Unknown
Epiphora Unlikely Related No None Ongoing
Corneal Abrasion Unlikely Related No Medication Resolved
LipiFlow
(Cohort 2)
Foreign Body Sensation Possibly Related No Medication Resolved
Dry Eye Disease Possibly Related No None Resolved
Foreign Body Sensation Possibly Related No None Resolved
Decrease in BCVA Definitely Unrelated No None Ongoing
Ocular Pain Unlikely Related No None Resolved
2. TEARCARE SYSTEM INFORMATION
The secondary safety endpoints included measurement of study
subject discomfort and pain during and after treatment, change
in best corrected visual acuity (BCVA) and change in intraocular
pressure (IOP).
There were subjects in both groups reporting pain and discomfort
during and after the respective procedures. Subjects in the TearCare
group initially reported higher pain/discomfort than LipiFlow
subjects during and immediately following the procedure. However,
by Day 1 the reported pain and discomfort was reduced and TearCare
results were less than LipiFlow. Subjects were asked to indicate their
level of pain and discomfort using a Visual Analog Scale with “0”
indicating no pain/discomfort to “100” indicating worst or maximum
pain/discomfort, as shown in Tables 8 and 9 below.

10
Table 8. Proportion of subjects reporting pain, stratified by treatment arm and cohort
TearCare LipiFlow
Pain
Thresholds
During
Procedure
N (%)
During
Expression
N (%)
After
Procedure
N (%)
1 day after
procedure
N (%)
During
Procedure
N (%)
After
Procedure
N (%)
1 day after
procedure
N (%)
Cohort 1 (n=46) Cohort 1 (n=47)
0-39 43 (93.5%) 32 (69.6%) 44 (95.7%) 44 (95.7%) 45 (95.7%) 47 (100.0%) 45 (95.7%)
40-69 2 (4.3%) 12 (26.1%) 2 (4.3%) 1 (2.2%) 2 (4.3%) 0 (0.8%) 2 (4.3%)
70-100 1 (2.2%) 2 (4.3%) 0 (0.0%) 1 (2.2%) 0 (0.0%) 0 (0.0%) 0 (0.0%)
Cohort 2 (n=69) Cohort 2 (n=73)
0-39 63 (91.3%) 49 (71.0%) 65 (94.2%) 67 (96.5%) 72 (98.6%) 72 (98.6%) 70 (95.9%)
40-69 4 (5.8%) 16 (23.2%) 4 (5.8%) 2 (2.9%) 1 (1.4%) 1 (1.4%) 2 (2.7%)
70-100 2 (2.9%) 4 (5.8%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 1 (1.4%)
Table 9. Proportion of subjects reporting discomfort, stratified by treatment arm and cohort
TearCare (n=115) LipiFlow (n=120)
Discomfort
Thresholds
During
Procedure
N (%)
During
Expression
N (%)
After
Procedure
N (%)
1 day after
procedure
N (%)
During
Procedure
N (%)
After
Procedure
N (%)
1 day after
procedure
N (%)
Cohort 1 (n=46) Cohort 1 (n=47)
0-39 34 (73.9%) 21 (45.7%) 42 (91.3%) 41 (89.1%) 37 (78.7%) 47 (100.0%) 40 (85.1%)
40-69 11 (23.9%) 17 (37.0%) 4 (8.7%) 4 (8.7%) 10 (21.3%) 0 (0.0%) 6 (12.8%)
70-100 1 (2.2%) 8 (17.4%) 0 (0.0%) 1 (2.2%) 0 (0.0%) 0 (0.0%) 1 (2.1%)

11
TearCare (n=115) LipiFlow (n=120)
Pain
Thresholds
During
Procedure
N (%)
During
Expression
N (%)
After
Procedure
N (%)
1 day after
procedure
N (%)
During
Procedure
N (%)
After
Procedure
N (%)
1 day after
procedure
N (%)
Cohort 2 (n=69) Cohort 2 (n=73)
0-39 58 (84.1%) 36 (52.2%) 64 (92.8%) 61 (88.4%) 63 (86.3%) 71 (97.3%) 56 (76.7%)
40-69 9 (13.0%) 27 (39.1%) 5 (7.2%) 7 (10.1%) 9 (12.3%) 2 (2.7%) 13 (17.8%)
70-100 2 (2.9%) 6 (8.7%) 0 (0.0%) 1 (1.4%) 1 (1.4%) 0 (0.0%) 4 (5.5%)
2. TEARCARE SYSTEM INFORMATION
One subject in the Cohort 1 and one subject in Cohort 2 of
TearCare group and one in the Cohort 2 of LipiFlow group
reported a decrease in visual acuity during the study. One subject
treated in the Cohort 1 of TearCare group had a history of visual
fluctuation in the right eye. The loss of visual acuity was reported
at 2-weeks which was recovered at 1-month visit. A second
TearCare study subject treated under Cohort 2 experienced loss
of 10 letters at two weeks following treatment and the visual
acuity further was reduced by 15 letters at one month compared
to baseline. All other ocular findings for this subject were within
the normal limits. The investigator suspects an error in visual
acuity measurement and reported that it is highly likely that the
uncorrected visual acuity was measured in place of best corrected
visual acuity. Both AEs were categorized as “unrelated to device
or procedure”. One subject treated in the Cohort 2 of LipiFlow
group had a history of fluctuating vision in the left eye. The subject
read 20 letters at baseline, 30 at 2-weeks and 10 at 1-month.
The investigator did not consider this AE as device or procedure
related. No other subjects reported any significant visual acuity
change in either group compared to baseline.
The overall safety results are similar between the TearCare System
subject device and the LipiFlow System predicate device with
respect to the safety profile.

12
2.8 DEVICE DESCRIPTION
The TearCare System is designed to deliver controlled, precise heat
to the tarsal plates and underlying meibomian glands of the eyelids
for 15 minutes. The TearCare System is comprised of a re-usable
SmartHub™ and accessories, and single use SmartLids®.
The TearCare System is comprised of the following components:
MODEL NUMBER DESCRIPTION
5-116
5-101
VEP15US09
5-102
TearCare SmartHub Kit, which includes:
TearCare SmartHub
Charging Adapter (XP Power
P/N VEP15US09)
Charging Nest
5-117 One (1) packaged pair of single-use
TearCare SmartLids
The single use SmartLid pair comprises four flexible, sensor-
controlled strips that adhere to each of the four eyelids. They
contain flexible circuits, sensors and a microprocessor which
provide accurate and precise thermal energy to the eyelids to melt
oil in the meibomian glands. Medical grade adhesive on the skin-
facing surface of the SmartLids allow them to be axed to the
external surface of the eyelids during the procedure and
easily removed at the end of the procedure.
The SmartLids are connected to the SmartHub. When attached to
the SmartHub, the SmartLids deliver thermal energy (i.e., heat) to
the eyelids. Embedded software and a closed loop sensor system
ensures that the temperature delivered at the eyelids is maintained
within a precise range. The user can adjust the temperature using
control buttons on the SmartHub.
2.8.1 TEARCARE SMARTHUB
The SmartHub, shown in Figure 1 below, is a battery-powered unit
that powers and controls the SmartLids. It features a circuit board,
microprocessor, ports for receiving the SmartLid connector(s), and
a port for connecting to the charging nest. The SmartHub features
a built-in rechargeable Li-ion battery that, when fully charged,
supplies power for at least 4 therapies. The SmartHub may be
used with one or two SmartLid devices connected. The SmartLid
connectors are inserted into the SmartHub’s device ports (6a, and/
or 6b).
A control button on the center of the SmartHub (1) is used to turn
the system on and o, and to initiate or discontinue the TearCare
session. Two buttons, “+” and “-”, on the SmartHub (2 and 5) allow
the user to adjust the preferred temperature level. The SmartHub
also has a display on the right side of its face that indicates how
much time is left in the session (10 and 11).

13
Figure 1. TearCare SmartHub Feature key
NOTE FUNCTION
1. Power button
2. Warmth setting increase button
3. Warmth setting indicator #4 (of 5)
4. Active therapy indicator
5. Warmth setting decrease button
6a. SmartLid Port - left
6b. SmartLid Port - right
7. Charging Port
8a. SmartLid error indicator - left
8b. SmartLid error indicator - right
9. Remaining Procedures (battery indicator)
10. Timer complete indicator
11. Timer indicators
2. TEARCARE SYSTEM INFORMATION

14
2.8.2 TEARCARE SMARTLIDS
SmartLids are flexible, software and sensor-controlled, single-use
heat treatment components. The SmartLids are connected via a
4 foot cable to the SmartHub, which provides power to heat and
regulate the SmartLid temperature.
Each SmartLid is comprised of one cable, one temple pad, and two
flexible curved elements. A Left and Right SmartLid are provided
in a single package so that all four eyelids may be heated in a
single session. The SmartLid delivers heat in a preset temperature
range, and ramps from approximately 41oC to 45oC when the
procedure is initiated. A thin adhesive holds the SmartLid on the
patient’s eyelids and temple. A clip is included with each SmartLid.
This clip may be used to secure the cables behind the patient’s
head.
SmartLids are digitally marked as “used” during a session and
cannot be re-used. The SmartHub will not allow initiation of a
session with a used SmartLid in place.
Cable Temple Pad Hinge Section
SmartLid™
flexible elements
Figure 2. TearCare SmartLid

15
3.1 UNPACKING AND CHECKING THE TEARCARE SYSTEM
PRODUCTS
Warning: Do not use any TearCare component if the
component or its packaging appears damaged. Inspect all
components for damage before each use.
Caution: Do not use SmartLids if they are expired (past the
expiration date on the label).
a) Tear the perforated strip o and remove the tray containing the
Smarlids from the shelf carton.
b) Remove the SmartLids from the tray one at a time. Remove the
cable first, starting with the connector, and carefully unwind
the cable.
c) Turn on the SmartHub by pressing the “Power” button for 1
second.
i. At least one of the Remaining Procedures (battery level)
indicators should be lit to indicate the battery has sucient
charge to complete a procedure.
ii. The amber “L” and “R” lights should be lit to indicate no
cables are plugged in (Figure 3, left image).
d) Plug each cable into the SmartHub.
e) Confirm that the SmartHub recognizes the cables by observing
the amber “L” and “R” lights going o (Figure 3, right image).
Ifthe amber light does not go o, refer to Troubleshooting
(Section 7).
f) Unplug the cables from the SmartHub.
Figure 3. Status of SmartLids: No SmartLids plugged in (Left); Both SmartLids
plugged in and functional (Right)
3.2 PREPARING THE PATIENT
Caution: Ensure the patient’s skin is clean and free of make-up,
oil or other contaminants. SmartLids are axed to the eyelids
with adhesive and will not adhere well to oily skin or make-up.
Poor adherence of the SmartLids to the skin may result in an
error message and temporary pausing of the procedure.
a) Prior to applying the SmartLid, clean the patient’s eyelids and
temples with a non-moisturizing make-up removal wipe to
remove any make-up, oil, dirt or lotion.
b) After cleaning, ensure the patient’s skin is dry before applying
any portion of the SmartLid.
3. PERFORMING A TEARCARE PROCEDURE

16
Caution: Eyelid cleansing may lead to skin irritation, which
would put a patient at increased risk for thermal injury. If the
patient is observed to have skin irritation, or if the patient
complains of skin irritation after eyelid cleaning, do not
proceed with the TearCare treatment.
c) If the patient is wearing contact lenses, have them remove their
lenses prior to the TearCare procedure.
3.3 APPLYING THE SMARTLID
Caution: Remove the SmartLid adhesive liners slowly to
ensure they do not become damaged during the application
process.
Caution: Do not position the SmartLids on top of the
eyelashes.
Caution: Ensure the SmartLids are fully installed, completely
contacting the patient’s eyelid skin, and stationary.
The SmartHub may prevent/pause the procedure if the
SmartLids are not fully in contact with the eyelid skin.
a) The SmartLids are designed to fit either the left or right eyelids
and are marked with an “L” or “R” next to the temple housing.
Apply the SmartLid to the external eyelid surface one eyelid at
a time, matching the “L” and “R” to the corresponding eyelid.
b) Applying the SmartLid to the Upper Eyelid:
i. Carefully remove the liner from the Upper SmartLid to reveal
the adhesive. Peel from the temporal tab towards nasal and
discard the liner.
ii. Ensure the SmartLids are adequately curved to conform to
the patient’s eyelid. To the extent needed, further curve the
SmartLids by forming a bend at the distal tip, and another in
the middle portion.
iii. Have the patient tilt their head slightly up, and look down-
ward with eyes closed.
iv. The goal is to apply the upper SmartLid so that it is angled
upward toward the center of the brow. It will straighten once
the temple housing is attached.
v. Apply the nasal end of the upper SmartLid just lateral to the
punctum, approximately 3-4 mm from the lid margin.
vi. Apply the lateral end about 1-2 mm from the lid margin.
c) Applying the SmartLid to the Lower Eyelid:
i. Carefully remove the liner from the Lower SmartLid to reveal
the adhesive. Peel from the temporal tab towards nasal and
discard the liner, ensuring not to disrupt upper lid.
ii. Have the patient tilt their head slightly down look upward
with eyes closed.
iii. Apply the nasal end just lateral to the punctum.
iv. Apply the SmartLid parallel to the lid margin, approximately
1-2 mm from the margin.
v. Ensure that SmartLids are properly adhered to patient’s
eyelids
Table of contents
Popular Receiver manuals by other brands

Panasonic
Panasonic RF-B65D Service manual

thomann
thomann the t.bone Tour Guide Talkback Receiver quick start guide

Pioneer
Pioneer Elite VSX-55TXi Service manual

RoboGuard
RoboGuard uRx manual

Scientific Atlanta
Scientific Atlanta Cox Business Video Digital Receivers user guide

Hemisphere GPS
Hemisphere GPS 874-0004-000 quick start guide











