i
Table of Contents
SECTION 1 - INTRODUCTION........................................................................................................1
1.1 DenitionsandSymbols.......................................................................................................1
1.1.1 Denitions ....................................................................................................................1
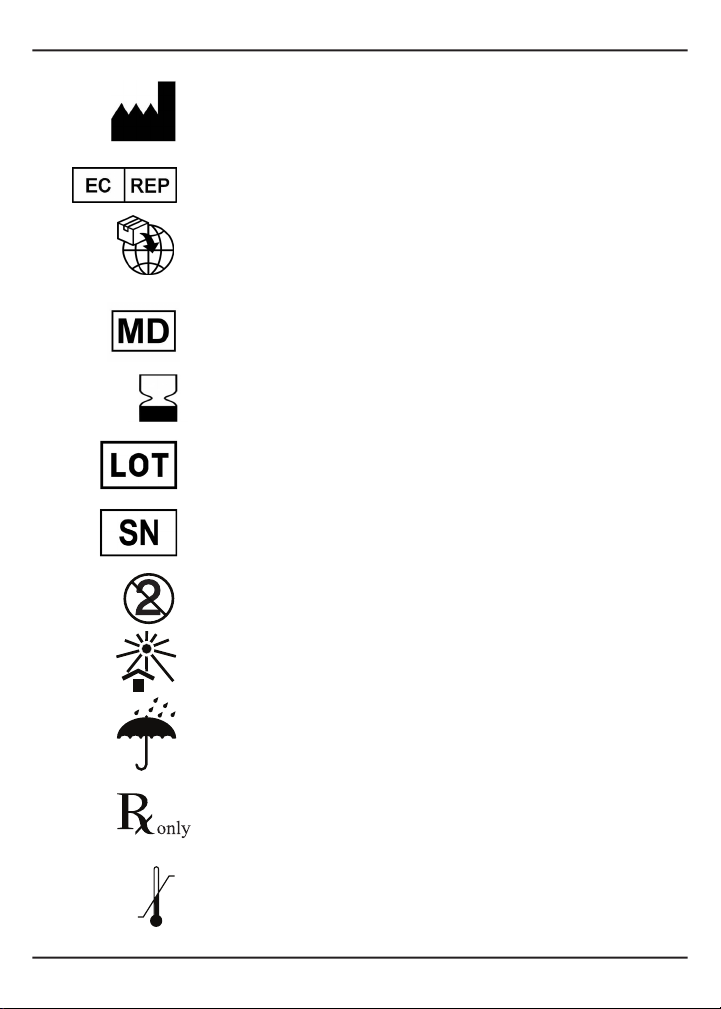
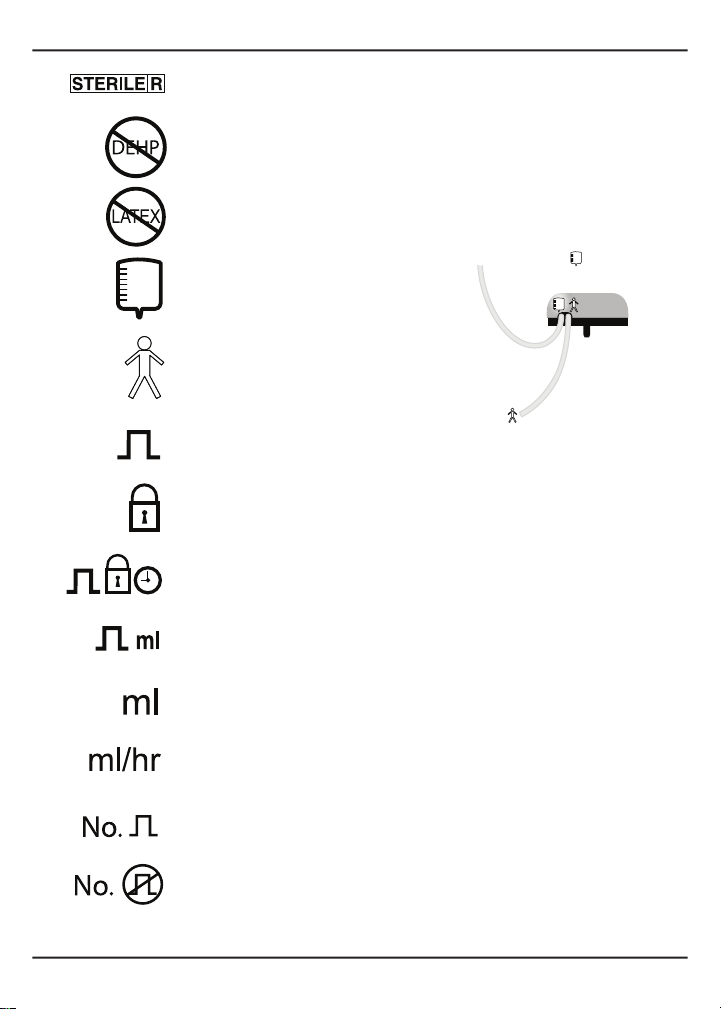
1.1.2 DenitionofSymbols..................................................................................................2
1.2 Warnings.................................................................................................................................6
1.2.1 Sterile, Disposable (Single-Use) Administration Set ..............................................8
1.2.2 Protection From Air Infusion.....................................................................................8
1.2.3 Protection From Unintended Bolus ..........................................................................8
1.2.4 Use of ambIT®Pump in MRI Environment .............................................................8
1.3 Indications for Use.................................................................................................................9
1.3.1 Continuous Pumps......................................................................................................9
1.3.2 PreSet Pumps ...............................................................................................................9
1.3.3 PCA Pumps ..................................................................................................................9
1.3.4 PreSet*PCA Pump .......................................................................................................9
1.4 Overview.................................................................................................................................9
1.4.1 Continuous Pumps....................................................................................................10
1.4.2 PreSet Pumps .............................................................................................................10
1.4.3 PCA Pumps ................................................................................................................10
1.5 Infusion Patterns..................................................................................................................11
SECTION 2 - SET UP .........................................................................................................................13
2.1 Required Materials ..............................................................................................................13
2.2 ambIT
®
Cassette ...................................................................................................................14
2.3 Priming the Cassette ...........................................................................................................14
2.4 Attach Cassette to the Pump..............................................................................................17
2.5 Remove Cassette from the Pump......................................................................................18
2.6 Changing the Fluid Reservoir............................................................................................18
2.7 Battery Installation and Replacement...............................................................................18
2.7.1 Battery Installation ....................................................................................................19
2.7.2 Battery Replacement .................................................................................................19
2.8 Pump Power On and Off....................................................................................................20
SECTION 3 - PROGRAMMING INSTRUCTIONS ....................................................................21
3.1 General Information............................................................................................................21
3.2 ambIT®Pump User Interface .............................................................................................22
3.3 Program Options .................................................................................................................23
3.4 Continuous Pump Programming Steps ...........................................................................23
3.5 PreSet Pump Programming Options ................................................................................24
3.6 PCA Pump Programming Steps........................................................................................29
3.7 Program Review...................................................................................................................31
SECTION 4 - OPERATING INSTRUCTIONS .............................................................................32
4.1 Start Infusion........................................................................................................................32
4.2 Pause Infusion......................................................................................................................33
4.3 Resume Infusion ..................................................................................................................33
4.4 Silence Alarm .......................................................................................................................33
4.5 Bolus Activation...................................................................................................................34
4.5a FUNCTION Button Activation..........................................................................................34
4.6 Summary of Operating Controls for Continuous Pumps .............................................35
4.7 Summary of Operating Controls for PreSet and PCA Pumps......................................35