3
4
-Pads should not touch each other when placed onto your skin.
-The materials (e.g. ABS) of expect contact with patient had passed the ISO 10993-5 and ISO
10993-10 standards test, no toxicity, allergy and irritation reaction. However, based on the
current science and technology, other potential allergic reactions are unknown.If you have
allergic reaction to materials, please stop treatment immediately and consult your physician.
-Keep the device out of the reach of children and pets to avoid inhalation or swallowing of small
parts. Do not allow children to take their temperatures unattended. Children may not be able
to use the device according to the instructions in this user manual. It is not a toy.
-TENS is not eective for pain of central origin, including headache;
-TENS is not a substitute for pain medications and other pain management therapies;
-TENS devices have no curative value;
-TENS is a symptomatic treatment and, as such, suppresses the sensation of pain that would
otherwise serve as a protective mechanism;
-Eectiveness is highly dependent upon patient selection by a practitioner qualied in the
management of pain patients;
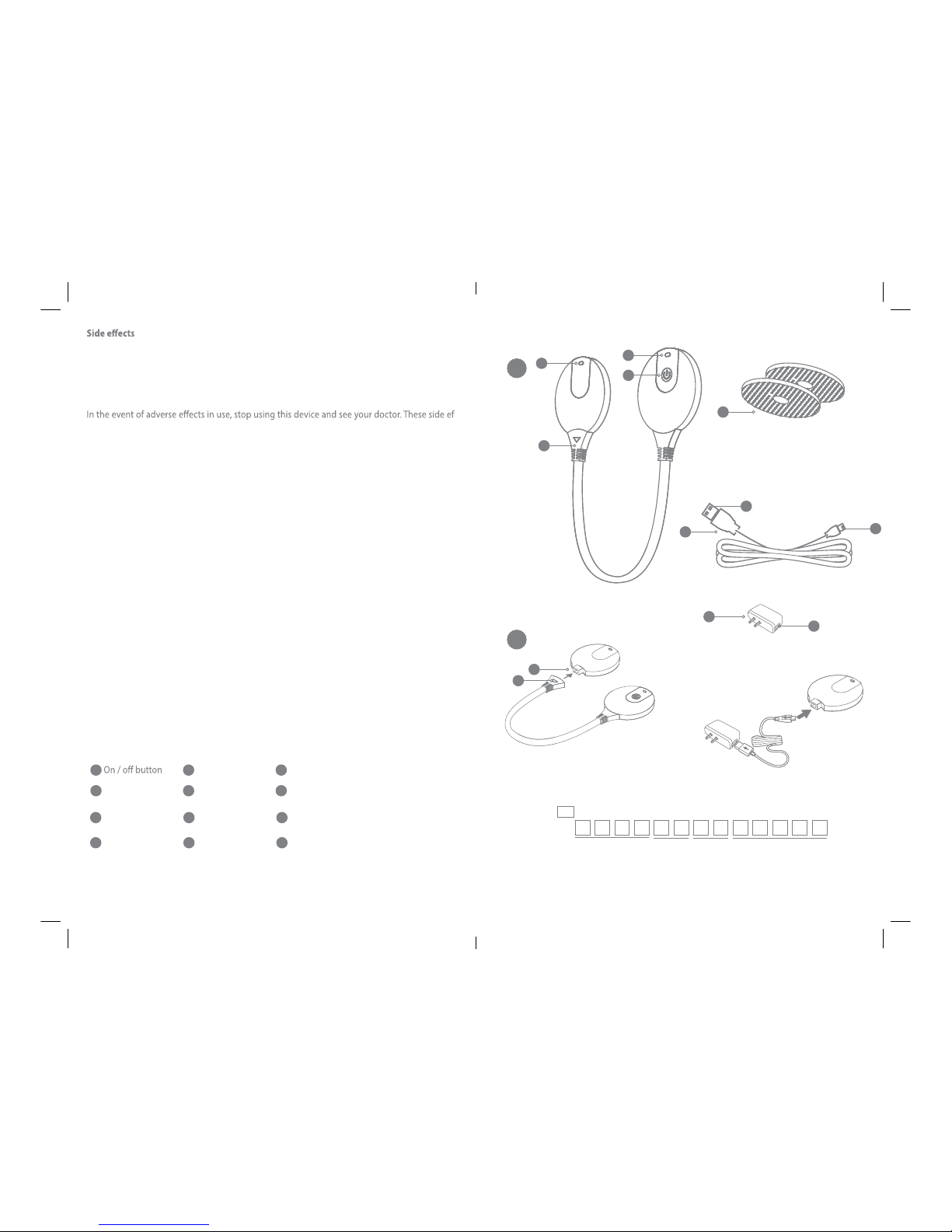
-Use this device only with the self-adhesive hydrogel electrodes,the adapter and USB cord
supplied with the device,which proved by manufacturer.
-This device complies with Part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) this device may not cause harmful interference, and (2) this device must accept
any interference received, including interference that may cause undesired operation.
Do not treat the the following parts or areas :
- D
-If you are in the care of a physician, consult with your physician before using this device;
-If you have had medical or physical treatment for your pain, consult with your
physician before using this device;
-If your pain does not improve, becomes more than mild, or continues for more than
ve days, stop using the device and consult with your physician;
-The long-term eects of electrical stimulation are unknown;
o not apply stimulation on your torso. Indeed, the introduction of electric current on this area
can cause heart rhythm disturbances, with a risk of death.
-
be avoided.
-
on your blood pressure or your heart rhythm.
- Do not position the electrodes on broken or injured skin, or which is dirty or unhealthy. Skin
with irritation, sores or other lesions can lead to the injection of too much current on the area,
which can cause burns.
- Do not place the electrodes near cancerous lesions because this may have a negative impact
on these injuries.
- Do not place the electrodes on skin areas whose sensations are not normal. You may burn
yourself due to a lack of feeling of the high intensity of the current.
-
(eg. phlebitis, thrombophlebitis and varicose veins). Stimulation should not be performed on
areas of thrombosis or thrombophlebitis because it can promote the circulation and lead to a
greater risk of embolism.
- Do not put the electrodes on redness or open wounds. Open wounds may lead to applying too
much current on the zone, causing burns. They can also favour the penetration of substances
from the electrode into the skin.
- Do not place the electrodes on the inside of body cavities, such as in the mouth. Indeed, this
device is only designed for external application.
-
-
-
Do not make sudden movements during a session. This could cause a dysfunction of the device.
Do not place the electrodes directly on the eyes,chest and the upper back or crossing over the
heart.
Do not use the device in the following conditions :
- Do not use the device if you are connected to high frequency surgical equipment. This could
lead to burns on the skin under the electrodes and damage the device.
- Do not use the electro-stimulator if you are monitored by a doctor and you have not consulted
him before using it.
- In the case of internal bleeding due to impacts or injury, do not use the device.
- To contract a muscle, do not use the electrical muscle stimulation in case of risk of muscle
contraction that can disrupt the healing process. If the tendon or the muscle is torn, a muscle
contraction can aggravate the wear, like a voluntary contraction. After recent surgery, after an
acute trauma or fracture, this situation can also happen. In case of occurrence of a tendonitis, a
muscle contraction can also aggravate the symptoms.
- Do not use the device while driving, operating the machines or any other activity during which
the electrical stimulation may lead to a risk of injury.
- Do not use the appliance if you are subject to falling asleep during the session, as this may
cause you to feel pain too late. If using at the time of goign to bed, set the timer so that the
- Never use the device in contact with water (in the bathroom, in the shower or in the pool, etc. )
because this increases the risk of an electric shock and skin burns.
-Do not apply stimulation over your neck because this could cause severe muscle spasms
resulting in closure of your airway, diculty in breathing, or adverse eects on heart rhythm
or blood pressure;
-Do not apply stimulation over, or in proximity to, cancerous lesions;