Innovatione 1S PRO User manual

1S PRO
Hair Removal Machine Instructions

Contents
Machine ........................................................................................................................................................4
Package.........................................................................................................................................................5
Technical Specications...................................................................................................................6
User manual ..............................................................................................................................................7
General information ...........................................................................................................................7
Precautions ................................................................................................................................................8
Contraindications ..............................................................................................................................11
Hair removal procedure recommendations .................................................................12
First use......................................................................................................................................................13
Turning on and o.............................................................................................................................14
Hair removal procedure................................................................................................................15
Machine cleaning and maintenance ..................................................................................16
Safety measures..................................................................................................................................17
Transportation and storage.......................................................................................................18
Resources, terms of service, and manufacturer’s warranty..............................19
Material information .......................................................................................................................20
Disposal .....................................................................................................................................................20
Certicate of acceptance and packaging........................................................................21
Preservation storage....................................................................................................................... 22
Revision record sheet .....................................................................................................................22

4
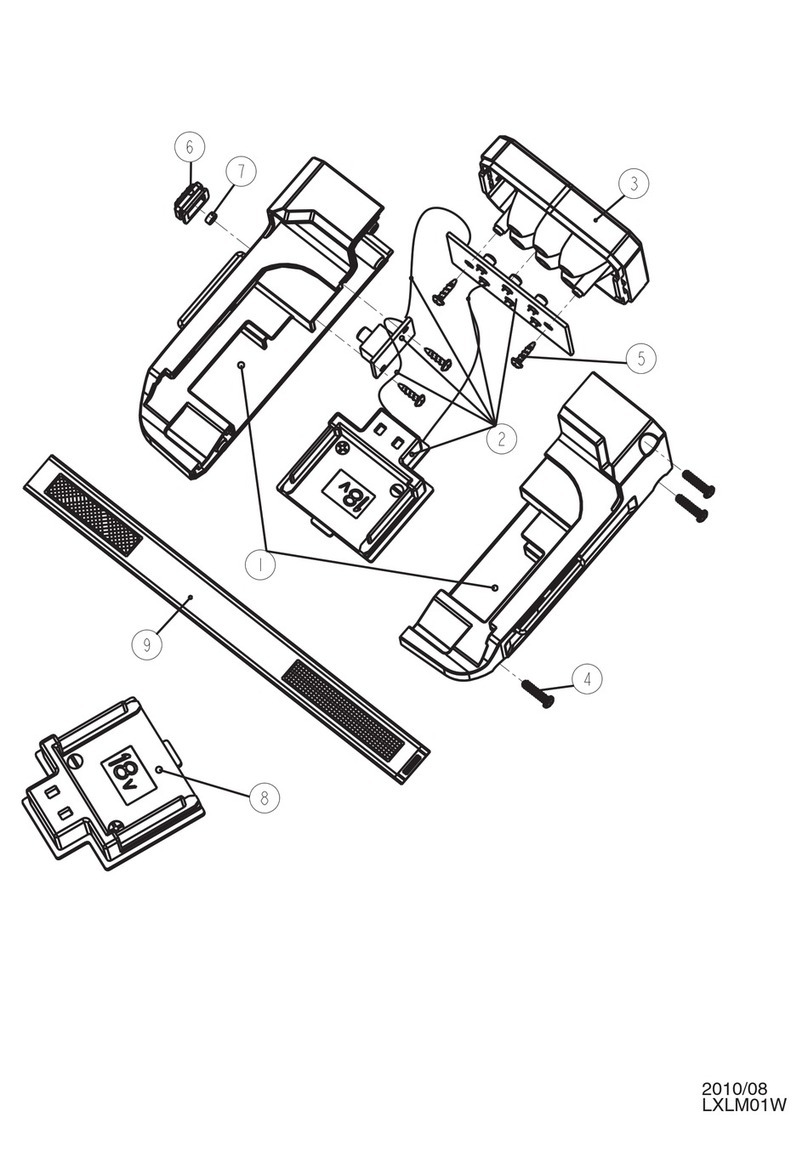
Machine
Applicator activation/
deactivation button
Applicator
Machine body
Power button
5
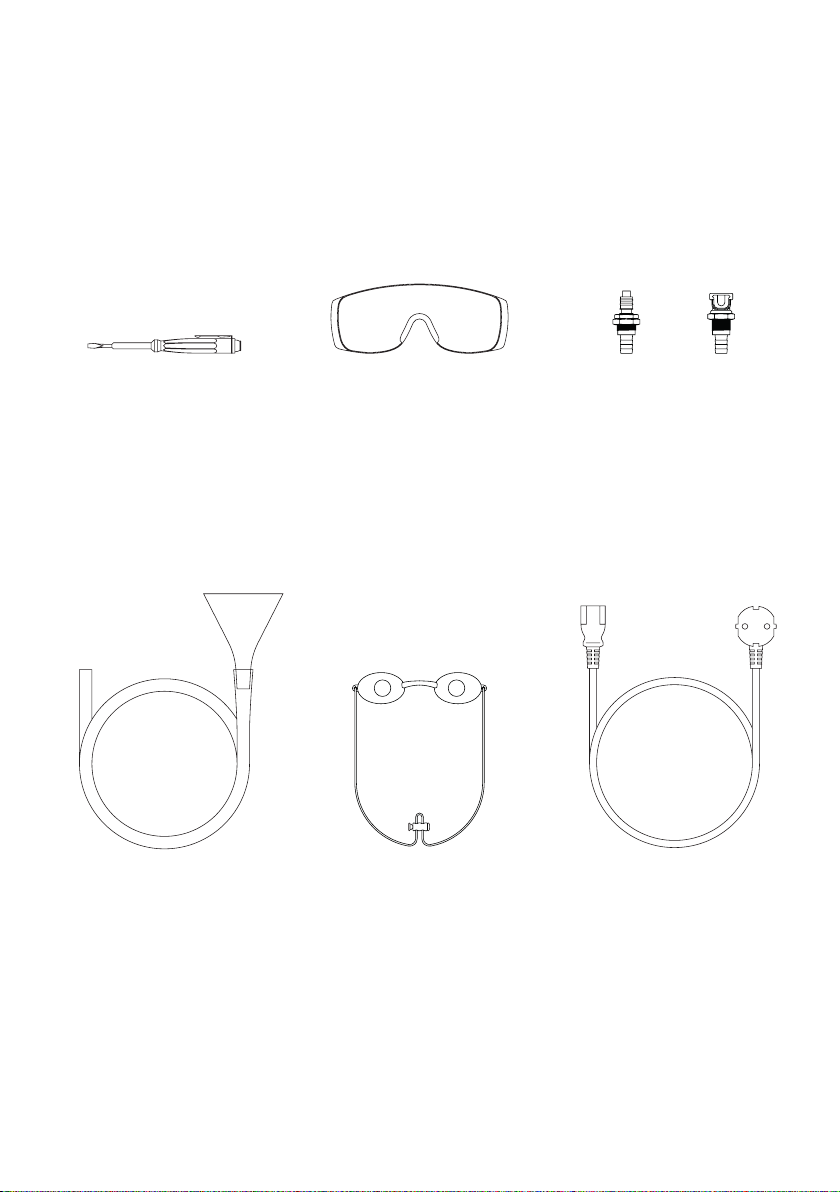
Package contents
Operator glassesMains tester
screwdriver
Valves to drain water
from the applicator
Customer glasses – 2 pcs Power cordFunnel

6
Technical specications
Professional Hair Removal Machine
Power consumption.............................................................................................................. 2000 W
Emitter resource...................................................................................................1,500,000 pulses
Safety sensors................................................................................................................................. 4 pcs
Package dimensions...................................................................................... 574/447/490 mm
Power supply...................................................................................................................................220 V
Cooling system.................................................................................................................Cooling Pro
System used ...............................................................................................................................IPLASER
Light wavelength............................................................................................700 нм – 1300 nm
Shipping weight........................................................................................................................22,5 kg
Developer............................................................................................................................Innovatione

7
User Manual
Attention! Prior to using the device, please read this User Manual carefully. If
you have any questions or experience any problems with the device, please
contact our Innovatione Customer Service Department by phone
+7 926 975 61 11.
If you have any questions or experience problems with the hair removal
procedure, please contact our Medical Department by phone
+7 926 547 03 33.
General information
Intended use: This product is intended to be used as a source of luminous
flux of a specific length to affect hair follicles.
Indications: The 1S PRO device is designed to remove hair and prevent their
subsequent growth. It is suitable for all 6 skin phototypes.
Buyers can order additional applicators, glasses, hoses with funnels, and
branded cleaning fluid from the manufacturer.

8
Precautions
1. Use this system strictly for its intended purpose as described in this
User Manual. Follow your doctor’s advice. Any other use of this device
is inappropriate and can be dangerous. The manufacturer is not liable
for any damage incurred due to device improper or unreasonable use
or if it was connected to the electricity mains incompatible with safety
requirements.
2. It is recommended to use the device with a quality 2,000 W mains
voltage stabilizer.
3. Prior to plugging the product into the mains outlet, make sure that the
mains voltage and frequency comply with the electrical specifications
shown on the specification plate on the back of the product.
4. The device must be used in a room temperature of 10 to 28o C.
5. If the device was exposed to conditions other than the acceptable
operating conditions, please wait until it restores its normal condition
before using it.
6. In case of the ingress of any extraneous liquids into the device, it must be
allowed to dry before use.
7. Do not use tap or purified water or other liquids not recommended by
the developer for the device system cooling.
8. Use the device in a clean environment free of dirt, dust, animal hair, etc.
9. The device should not be used near flammable substances or flammable
anesthetic mixtures with air, oxygen or premixed nitrous oxide.
10. It is forbidden to make any changes to the device design.
11. The device may heat up when used.
12. Do not place the device on top of any other equipment or under it. Do
not open the applicator body as it is not intended to be serviced.
13. Proper operation of this device may be impaired if you fail to follow these
User Manual instructions and/or do not use original spare parts.
14. Do not place the device on furniture and/or materials able to accumulate
static electricity.
15. While working with the device, the operator and the customer are
advised to avoid using things accumulating static electricity.
16. This device should be used intermittently in “1,000 pulses/10 min break”

9
mode. If used improperly, the device may be seriously damaged.
17. Do not place the device in water, as it is not water resistant.
18. Do not touch the device with wet hands.
19. Do not leave the device outdoors.
20. Place the device on a stable, horizontal surface at a distance of at least 4
cm from the walls.
21. Make sure that the air inlets in the device body are not clogged.
22. Do not allow disabled people or children to use the device.
23. Do not turn off the device by simply disconnecting its power cord from
the mains.
24. Do not use the device if it is damaged or its body has cracks.
25. It is recommended to have a spare applicator in case you cannot use the
applicator supplied (for example, when it was sent for replacement).
26. Do not use adapters (standard or with multiple connectors) or extension
cords.
27. Do not leave the idle device permanently connected to the mains.
Disconnect the device from the mains outlet if do not intend to use it for
a long time.
28. Follow the instructions when connecting the device parts. The
manufacturer is not liable for any damage caused by improper
connections.
29. Use the power cord supplied. The user is not allowed to replace
the power cord. In case the power cord is damaged, please contact
Innovatione Service Department by phone +7 926 975 61 11 to replace
the cord (the cord cannot be repaired).
30. Prior to any cleaning procedure or replacing the applicator, turn off the
device and disconnect it from the mains.
31. Clogged air inlets in the device body or applicator, dirty glass or improper
connections of the device parts may cause the device malfunction or
damage which is not a warranty case.
32. Do not expose the device to shocks and drops.
33. It is forbidden to open the device body, disassemble it, or repair it
yourself, or entrust the repairs to unqualified personnel without specific
authority for this activity type.
34. Do not apply excessive force to the device control buttons and its

10
structural elements when using it.
35. It is forbidden to expose the device to fire, moisture, or corrosive liquids
(alcohol, acids, alkalis, organic liquid substances, etc.).
36. Do not direct the applicator glass into the eyes. During the procedure,
make sure to use the protective glasses included in the package. It is
forbidden to use the device without protective glasses.
37. Keep this User Manual for future reference.

11
Contraindications
1. Epilepsy.
2. Pregnancy.
3. Lactation.
4. Hyperthermia.
5. Photodermatosis.
6. Oncology diseases.
7. Varicose veins.
8. Diseases, the progression of which may be affected by light waves.
9. Somatic diseases in the acute stage.
10. Weakened immune system.
11. Blood-clotting disorder or the use of drugs reducing blood clotting
(anticoagulants).
12. Acute infectious diseases.
13. Damaged skin on the application area.
14. The herpes virus in the acute stage.
15. Metal implants in the application area.
16. Neoplasms on the skin, malignant tumors.
17. Cochlear implants and/or pacemaker use.
18. Photosensitizing drugs use.
19. Diabetes mellitus in the decompensation stage.
20. Acute exacerbation of chronic diseases (systemic lupus erythematosus,
porphyria, eczema, psoriasis, lichen planus, ichthyosis, atopic dermatitis,
lupus erythematosus, scleroderma, bullous dermatosis, collagenosis,
vasculitis, and dyschromia).
21. Ischemic heart disease and hypertension.
22. In case of Botulinum toxin type A injections (Botox, Dysport, Xeomin,
Relatox), as well as various hyaluronic acid-based dermal fillers and
biorevitalizants injections, laser hair removal procedures can be carried
out in the injection areas no sooner than in 2 months after the injections
were administered.

12
Hair removal procedure
recommendations
1. The recommended treatment course is 6 to 10 sessions.
2. It is prohibited for the patient to use any type of peeling and scrubs for 3
days before and 3 days after the hair removal procedure.
3. Exclude exposure to direct sunlight and/or solarium light a week before
and after the hair removal procedure.
4. Exclude hair removal and depilation in the light wave application skin
areas a month before the procedure.
5. It is recommended to shave the area to be epilated one day before the
session.
6. Practical skills are perfected in the Innovatione training center. You can
sign up for the courses using the innovatione.ru website.
7. The luminous flux from the device affects melanin. Consequently, it is
more efficient when applied to dark hair.
8. It is prohibited to use the device without operators’ prior training in the
Innovatione training center.
9. Refrain from visiting baths, pools, saunas, or hot baths a day before and a
day after the hair removal procedure.
10. The treatment course does not guarantee the complete removal of
unwanted hair.

13
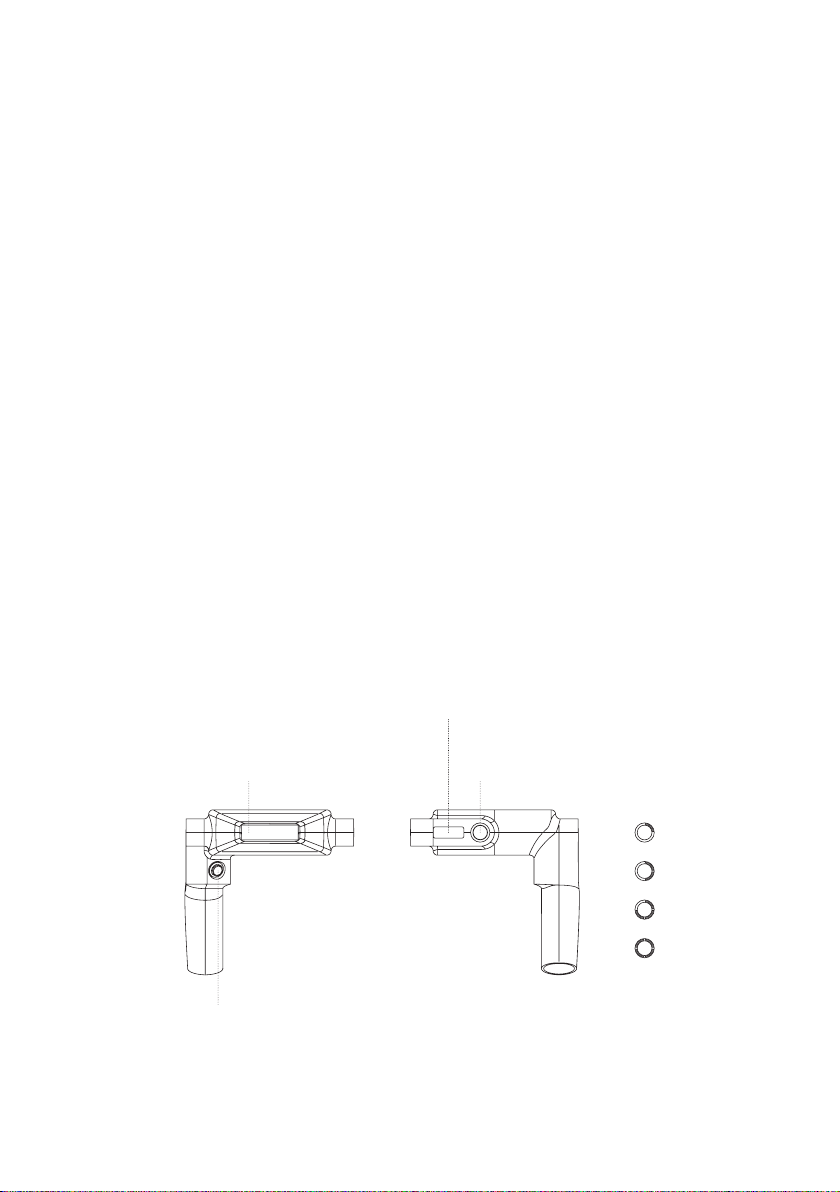
First use
1. After unpacking the system, check the device and all available
accessories for damage or defects, for example, device plastic body cracks
which may expose electrical components. Contact Innovatione Technical
Service or your supplier to report any damaged component.
2. Prepare 3 liters of distilled water.
3. Unscrew the two covers marked with numbers 1 and 2.
4. Insert the funnel hose into hole 1.
5. Pour water into the funnel until it flows out of hole 2.
6. Screw the covers back on.
7. The water needs to be changed every month.
8. To drain the water, you will have to unscrew the two covers marked with
the numbers 2 and 3. Before that, place a suitable 3-liter container under
the holes.
9. When the device was stored for a long time, it is strongly recommended
to replace the water in it before use.
10. It is strictly prohibited to transport the device with water in the cooling
system.
11. Plug the power cord into the mains outlet and hold its other end in
your hand. Insert the supplied mains tester screwdriver into hole L. If the
screwdriver diode is lit, the power cord is connected correctly. Otherwise,
reverse the power plug in the mains outlet.
12. Plug the power cord into the device power socket.
1
2
34
L
14
Turning on and o
1. Make sure the device is connected to the mains.
2. Press the on/off button and the cooling system fans start working.
3. Press and hold down the applicator activation/deactivation button until
you hear a beep sound and the power mode indicator on the applicator
lights up.
4. Power mode 1 is selected by default.
5. The device power control is carried out using the power mode selector.
6. When pressing the power mode selector, you can select the luminous
flux mode from 1 to 4.
7. The device is ready to use. Do not direct the applicator glass into the
eyes. When the release button is pressed once, three light impulses
emanate from the glass on the applicator.
8. To turn off the device, first, deactivate the applicator by holding the
activation/deactivating button on it until you hear a beep sound. After
that, press the device on/off button. The fans and the cooling system will
turn off.
Release button
Applicator glass Power mode selector
Impulse counter
1
2
3
4

15
Hair removal procedure
1. Before the procedure, please let the customer read the Informed Consent
Document.
2. Position the customer comfortably on the couch.
3. Put on gloves (latex, vinyl, or nitrile), a mask, and a cap.
4. Disinfect the protective glasses supplied in the package.
5. Put on protective glasses and get the customer to wear protective
glasses as well.
6. Disinfect the device applicator, using an antiseptic agent.
7. Prepare the body area intended for the hair removal procedure:
• if the hair length exceeds 1 to 2 mm shave the area with a trimmer, but
not with a razor;
• disinfect the area intended for hair removal using a skin antiseptic
agent (Estilodez, Diaseptic 30, etc.)
8. Turn on the machine and activate the applicator;
9. Release the first pulse sidewise, avoiding eye contact;
10. Start the procedure with power mode 1;
11. The laser working spot should be firmly against the skin during the
procedure;
12. Press the release button once to generate three pulses;
13. Position the laser working spot to evenly treat the area intended for hair
removal;
14. Handle the applicator, moving it a little to the previously treated area;
15. It is not recommended to treat the same area more than once.

16
Device cleaning and
maintenance
1. Before cleaning, make sure the power cord is disconnected from the
mains.
2. Wipe the device body with a clean, damp cloth at least once a month.
3. When needed, clean the device ventilation openings, including those
located on the lower part of the device to prevent overheating.
4. The applicator needs replacing when 1,500,000 pulses are reached.
5. The applicator must be disinfected with an antiseptic agent before and
after each procedure.
6. Replace the distilled water in the cooling system once a month. See the
“First Use” section to learn more about the replacement procedure.
7. Once in three months, pour in a proprietary cleaning liquid for 10 days
instead of distilled water.
8. Repairs, performed by unauthorized personnel will void the warranty.
9. In the full range of issues, contact your authorized supplier or Innovatione
Service Department.

17
Safety measures
The 1S PRO hair removal machine safety is ensured when designed and
manufactured in compliance with safety requirements specified in:
1. Directive 2014/35/EU “Low Voltage Equipment”.
2. Directive 2014/30/EC “Electromagnetic Compatibility”.
3. The Customs Union Technical Regulations TP TC 004/2011“On low-
voltage equipment safety”.
4. The Customs Union Technical Regulations TP TC 020/2011“Technical
equipment electromagnetic compatibility”.
5. GOST 12.2.007.0-75 “Occupational safety standards system. Electrical
products. General safety requirements.”
6. GOST 30805.14.1-2013 “Technical equipment electromagnetic
compatibility. Household appliances, electrical tools, and similar devices.
Industrial RF interference. Measurement regulations and methods.”
7. GOST 30805.14.2-2013 “Technical equipment electromagnetic
compatibility. Household appliances, electrical tools, and similar devices.
EMI resistance. Testing requirements and methods.”

18
Transportation and storage
1. The packaged device is transported by any type of enclosed transport,
including air transport, in heated sealed compartments in compliance
with shipping rules applicable to each transport type.
2. Devices transportation in containers is allowed.
3. During handling and transportation, the packaged device should not be
subjected to sharp shocks or exposed to precipitation.
4. When loaded for transportation, the packaged device should be
arranged the way which excludes its movement in the compartment
during transportation.
5. Transportation conditions must comply with storage conditions
according to GOST 15150-69.
6. Storage conditions must comply with GOST 23216-78 and GOST 15150-
69. Store the device in its package in a dry, heated, and ventilated room
with air temperature from 10 to 55° C at a relative humidity of not more
than 95%. The ambient air should not contain aggressive impurities.
7. When storing the device, it is necessary to ensure its inaccessibility to
children and animals.

19
Resources, terms of service, and
manufacturer’s warranty
1. The applicator (emitter) resource is 1,500,000 pulses. After 1,500,000
pulses are reached, contact the company’s managers to purchase a new
applicator.
2. The device service life is 7 years. The device can be stored in a dry heated
room for up to 1 year, provided that it is stored in factory preservation
condition.
3. The manufacturer provides a 24-month warranty servicing from the date
of sale, subject to the consumer’s compliance with instructions stipulated
in this User Manual.
4. If any manufacturing defects are revealed, the consumer should contact
their authorized supplier or Innovatione Service Department.
5. Malfunctions revealed in the device by the consumer during the
warranty period are eliminated for free by the manufacturer or its
authorized representative.
6. The manufacturer shall not be liable for any device malfunctions during
the warranty period if they are caused by:
• the consumer non-compliance with instructions stipulated in this User
Manual;
• the device careless storage and transportation by both the consumer
and the trading company;
• unauthorized device disassembly.

20
Material information
The device body and applicators: ABS plastic.
Sealed glasses: polyurethane.
Hose: rubber.
Laser working spot: glass.
Handpiece and funnel: aluminum.
Disposal
When the device is tested, stored, transported, operated and disposed of, it
does not exert chemical, thermal, radiation, electromagnetic, or biological
effects on the environment and does not require the use of environmental
protection agents from the effects specified. In case the device has worn out
and become unrepairable, contact a specialized service company to dispose
of it.
21
Acceptance and packaging
certicate
1S PRO hair removal device is complete and packaged into a shipping
container in accordance with the technical documentation requirements.
___________ _________ ________________ ____________
position signature decryption date
МП
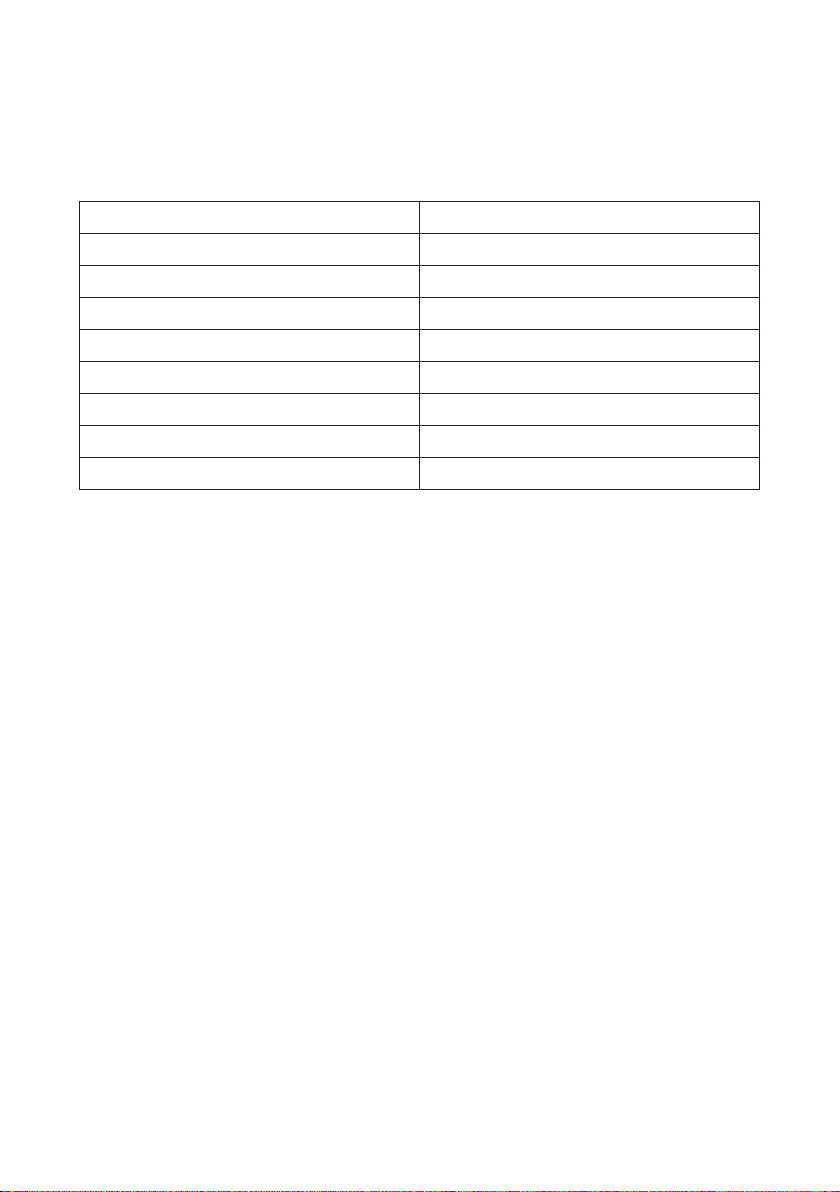
Product Name Serial Number
1S PRO device
Applicator
Table of contents
Popular Other manuals by other brands

aquaHabitats
aquaHabitats MicroHabitat 30 Instructions for use

BraunAbility
BraunAbility Driver Test Station user manual

STEIN
STEIN EMBRACE FRAMELESS HINGED DOOR - TILE installation instructions

Aitronic
Aitronic AT-120 operating manual

Whirlpool
Whirlpool W10120482A installation instructions

Hagen
Hagen FLUVAL 05 Series manual