Meditech OXYGEN DEMAND VALVE User manual

111-0063-00 (Doc 556M) Page 1 of 8 ISSUE H
18/03/2021
INSTRUCTIONS FOR USE
OXYGEN DEMAND VALVE
PAGE
1. SYMBOLS 2
2. INTENDED USE OF DEVICE 2
3. TECHNICAL DESCRIPTION 2
4. WARNINGS 3
5. INSTRUCTIONS FOR USE 4
6. CLEANING AND DISINFECTION 5
7. MAINTENANCE 5
8. SERIAL NUMBER 6
9. PRODUCT LIFE SPAN 6
10. SPECIFICATION 6
11. SPARE PARTS 6
12. RELEVANT APPLICABLE STANDARDS 6
COMPANY DETAILS 8
READ THESE INSTRUCTIONS BEFORE USING THE EQUIPMENT

111-0063-00 (Doc 556M) Page 2 of 8 ISSUE H
18/03/2021
1. SYMBOLS
2.INTENDED USE OF DEVICE
The B.N.O.S. Meditech Oxygen Demand Valve is intended for the self-administration of Medical
Oxygen. Oxygen should only be used for short-term/temporary therapy by self-administration of the
Demand Valve user and under supervision of suitably qualified medical personnel. Please refer to any
rules or restrictions that may cover the use of Oxygen in the specific country of use of the device.
The B.N.O.S. Meditech demand valve is designed for use with either reusable (autoclavable) patient
inhalation masks or single use patient inhalation masks/mouthpieces. It is highly recommended that a
single use filter is used between the demand valve and the facemask/mouthpiece to minimise the risk
of contamination of the device and subsequent cross contamination between users. Please ensure that
single use filters/facemasks/mouthpieces are correctly specified for use with Oxygen.
Filters/facemasks/mouthpieces require a 22mm female connection complying with BS EN ISO 5356-
1:2015.
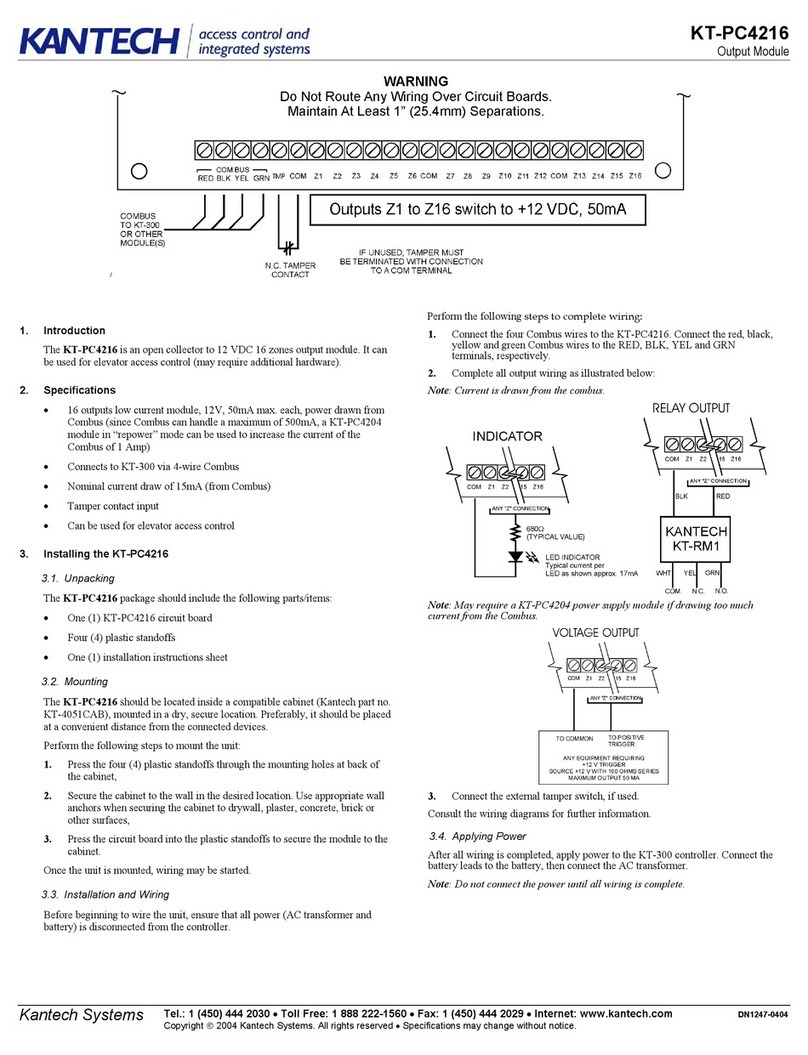
2. TECHNICAL DESCRIPTION
The principle of operation of the demand valve is similar to the original design of equipment used in the
diving market. A diaphragm (a) contained within the demand valve housing (b) acts upon a tilt valve
assembly (c). When the user exerts a negative pressure at the patient connection port (d) by inhaling,
the diaphragm actuates the tilt valve due to the negative pressure created within the housing
subsequently supplying a flow of gas at the patient connection of the device. (See Fig 1) The greater
the negative pressure exerted on the device, the higher the flow rate available at the patient connection
port. The flow rate is entirely related to the inspiratory effort of the user.
WARNINGS!
Indicates a potentially hazardous situation which could
result in injury to the user or others, if not avoided.
Read these instructions before using the equipment
Do not use any form of grease or oil
No Smoking
Do not use this device near any source of ignition
Product part number
Authorised Representative details
Medical device
Serial Number
Manufactured by
Next Maintenance or Service Due date

111-0063-00 (Doc 556M) Page 3 of 8 ISSUE H
18/03/2021
The demand
valve has
been
specifically
designed to
be used with low
cost single
use generic
mouth piece
and filter
systems that
are widely
available
using
standard
connections
that conform
to BS EN ISO 5356-1.

111-0063-00 (Doc 556M) Page 4 of 8 ISSUE H
18/03/2021
5. INSTRUCTIONS FOR USE
4. WARNINGS!
4.1 Please read these instructions before use. Do not use the demand valve if you do
not understand the instructions detailed in this user guide.
4.2 Do not use any form of grease or oil (hydrocarbon based substances) with
this device.
4.3 Do not use this device near any source of ignition e.g. naked flame, electrically
powered heaters, cigarettes etc.
4.4 Do not smoke any products including tobacco when using this device.
4.5 Medical Oxygen is a medicinal gas and its use must be supervised by correctly
trained medical staff and/or prescribed for use where applicable by a suitably
authorised medically trained individual e.g. Doctor.
4.6 Do not use the “Press to Flush/Test” button when the device is in use.
4.7 Ensure sufficient Oxygen is available for use before using the product.
4.8 If using medical gas cylinders, ensure that they are adequately secured or stowed
at all times.
4.9 The demand valve hose is fitted with medical gas specific connectors. Do not
interfere or modify the connector. Particular attention must be paid during any form
of maintenance to ensure that the correct medical gas specific connector is fitted to
the device.
4.10 If used with a medical gas regulator, always ensure that the regulator cylinder valve
is opened slowly.
4.11 The device must not be used with harness or retained face mask.
4.12 When using a regulator/cylinder, make sure that the regulator is capable of
providing the Demand Valve with a flow of at least 100 l/min @ 4 bar of gas, to
ensure that the device functions as intended. If in any doubt as to the suitability of
the medical gas regulator to be used with the device please contact the
manufacturer.
4.13 When the demand valve is not in use, turn off the regulator where appropriate and
disconnect the valve from the medical gas supply.
4.14 Check the demand valve and supply hose regularly for leaks. Remove any leaking
device from service immediately.
4.15 Ensure that the hose is always arranged in such a way that it cannot be damaged
and does not cause any form of obstruction or hazard.

111-0063-00 (Doc 556M) Page 5 of 8 ISSUE H
18/03/2021
5.1 Before connecting to a pressurised gas source, please ensure the unit is clean and in
good condition. If you have any doubts about the condition, please do not connect or use
the device. Please check that the inhalation diaphragm is fitted to the device and seated
correctly (laying flat against the body of the device).
5.2 Ensure that the supply hose attached to the demand valve is connected to an Oxygen
Medical Gas supply that uses the correct respective Medical Gas fitting.
5.3 If using an Oxygen regulator, please check that the cylinder has adequate gas and that
the cylinder valve is turned on. It is suggested that the portable cylinder is at least half full
and that additional cylinders are available if required.
5.4 Before administering Oxygen to the patient, please check the availability of gas by briefly
pressing the ‘Flus/test’ button, located on the top of the Demand Valve. Please check
that there are no audible sounds of gas flow once the ‘Flush/Test’ button has been
released.
5.5 It is strongly advised to use a single use filter with the Demand Valve to minimise the risk
of contamination of the device and a subsequent cross contamination between users.
Connect a universal patient inhalation mask or single use patient inhalation
mask/mouthpiece to the single use filter.
5.6 The Demand Valve is now ready for use. The user should be instructed to inhale through
the mouthpiece or face mask connected to the patient connection port fitted to the
Demand Valve. Doing so will create a negative pressure on the patient connection port
which will activate the supply of Medical Oxygen. The larger the breath taken by the user,
the greater the supply of Oxygen. Please advise the user not to press the ‘Flush/Test’
button whilst the device is in use.
5.7 The user is able to exhale through the mouthpiece or face mask.
5.8 The Demand Valve will stop supplying gas when the user stops inhaling. The exhaled
gas will exit through the Demand Valve via the exhalation port.
5.9 The device should continue to be used under the supervision of medically trained
professionals.
5.10 The duration of use of the device is to be determined by the medically trained
professionals who are supervising the use of the Demand Valve.

111-0063-00 (Doc 556M) Page 6 of 8 ISSUE H
18/03/2021
6. CLEANING AND DISINFECTION
6.1 Ensure demand valve is disconnected from the gas supply before cleaning.
6.2 Clean the device before first use and then subsequently after every use, ensure the
Demand Valve, including the handle is wiped over thoroughly with a disinfecting wipe. For
disinfection purposes a chlorine dioxide based product (eg the Tristel Wipes and solution
system) can be used, at a nominal concentration of 0.02% wt/vol. The concentration refers
to chlorine dioxide in water. Follow the manufacturer’s directions for safe use.
6.3 The Demand Valve is not suitable for autoclaving.
6.4 The Demand Valve should be thoroughly dried before storage.
7. MAINTENANCE
7.1 Maintenance must be carried out on the unit on a five yearly basis by B.N.O.S. Meditech
or engineers certified by B.N.O.S. Meditech. This activity involves dismantling the unit and
replacing all internal seals, diaphragm, medical gas supply hose and any components
which show significant wear and tear.
7.2 Performance should also be checked on a five yearly basis, using suitable test equipment.
The negative pressure required to generate specific flows from the device must be tested.
If required, B.N.O.S. Meditech Ltd. can advise on suitable test equipment.
7.3 For Demand Valves in frequent use, for example, an Ambulance Service or a Maternity
Unit, personnel trained to supervise the use of a Demand Valve should refer to 5.4 of this
Instruction For Use document and carry out the check as described on a regular basis. For
less frequent users, i.e. industrial safety use, a leak test should be periodically performed
by applying an oxygen compatible leak test solution to all medical gas supply hose fittings
and joints, where applicable. The suggested period is annually.
7.4 The medical gas supply hose MUST be changed every five years. Please contact the
manufacturer to determine the age of the hose fitted to the demand valve.
7.5 B.N.O.S. Meditech Ltd. offers training and certification on the service, repair and
preventative maintenance of its products.
8. SERIAL NUMBER
The Serial No. is to be found on the handle label of the demand valve. It consists of four sections,
the first letters expressing the general type of device, followed by numbers for the month and year of
manufacture and lastly a set of up to five numbers representing the individual “number” of the unit
and differentiating units built in the same month.
Example: ODV121700001
ODV 12 17 00001
Oxygen Demand Valve Month of Manufacture Year of Manufacture Number of Unit
9. PRODUCT LIFE SPAN

111-0063-00 (Doc 556M) Page 7 of 8 ISSUE H
18/03/2021
The Demand Valve is designed to have a product life span of 10 years, excluding abuse and/or
damage to the device.
10. SPECIFICATION
Inspiratory Resistance <1.5 kPa @ 200 l/min
<0.25 kPa @ 10 l/min
Expiratory Resistance <0.1kPa @ 12 l/min
<0.6kPa @ 120 l/min
Transport and Storage Temperature: -20°C to 60°C
Operating Temperature: 5°C to 40°C
Supply Pressure @ 100l/min Nominal 400 kPa
Maximum 600 kPa
Minimum 280 kPa
11.SPARE PARTS
Description Part Number
Inhalation Diaphragm 033-1031-00
12.RELEVANT APPLICABLE STANDARDS
B.N.O.S. Meditech Ltd is an ISO 13485:2016 registered company.
BS EN ISO 5356-1:2015 Anaesthetic and respiratory equipment, cones and connectors, cones and
sockets.
BS EN ISO 15001:2011 Anaesthetic and respiratory equipment. Compatibility with Oxygen.
BS EN ISO 5359:2014+A1:2017 Low pressure hose assemblies for use with medical gases.
BS 5682:2015 Probes (quick connectors) for use with medical gas pipeline systems.
BS EN ISO 14971:2019 Medical Devices. Application of risk management to medical devices.
BS EN ISO 15223-1:2016 Medical Devices. Symbols to be used with medical device labels,
labelling and information to be supplied. General requirements.
IMPORTANT NOTICE
Manufacturer’s Warranty is for a period of 5 years and includes parts and labour. It does not
include transport costs. The responsibility and cost of returning and collecting the unit from the
manufacturer or their authorised representative is the owners.
B.N.O.S Meditech Ltd reserves the right to change design without prior notice.
Any disassembly of the device beyond that detailed in this manual will invalidate the
warranty and the manufacturers disclaim any liability for products that have undergone
unauthorised repair.

111-0063-00 (Doc 556M) Page 8 of 8 ISSUE H
18/03/2021
COMPANY CONTACT DETAILS
This device is designed and manufactured by:
B.N.O.S. Meditech Ltd.
9 Fifth Avenue, Bluebridge Ind. Est.,
Halstead, Essex CO9 2SZ, England
Tel: +44 (0)1787 479475,
Fax: +44 (0)1787 477747
E-mail: sales@meditech.uk.com
www.meditech.uk.com
EC Representative
Medical Device Management
Block B, The Crescent Building
Northwood, Santry, Dublin 9,
Ireland D09 C6X8
2797
Table of contents
Popular Control Unit manuals by other brands

National Instruments
National Instruments NI 9882 operating instructions

Rain Bird
Rain Bird STP-400i Installation, programming & operation guide

HeadShot Controllers
HeadShot Controllers Arbiter 4 install guide

Honeywell
Honeywell Velociti AOM-2RF Installation and maintenance instructions

Swegon
Swegon TBLZ-1-48-1 Installation

OPTO 22
OPTO 22 SNAP-PID-V user guide