MESI mTABLET ABI User manual

Instructions
for Use
MESI mTABLET ABI
Ankle-Brachial Index
ISO 9001 Q-1664
ISO 13485 M-049
MTABSYSABI

CONTACT INFORMATION
Address MESI, development of medical devices, Ltd
Leskoškova cesta 11a
SI-1000 Ljubljana
Slovenia, European Union
Telephone +386 (0)1 620 34 87
E-mail [email protected]
Website www.mesimedical.com
DISTRIBUTOR INFORMATION

Instructions
for Use
ISO 9001 Q-1664
ISO 13485 M-049
MESI mTABLET ABI
Ankle-Brachial Index

CONTENT
1 SAFETY AND LEGAL RECOMMENDATIONS 6
1.1 LEGAL INFORMATION 6
1.2 SAFETY INFORMATION 6
1.2.1 Setup and technical personnel 6
1.2.2 Access to the device 6
1.2.3 Safety measures 6
2 PRODUCT DESCRIPTION 7
2.1 WHAT IS IN THE PACKAGE 7
2.1.1 Accessories 7
2.2 INTENDED USE 8
3 TECHNICAL SPECIFICATIONS 10
3.1 MESI TUBELESS CUFF UNIT (CUFFMD) 10
3.1.1 Dimensions 10
3.1.2 Power & battery 10
3.1.3 Cu sizes 10
3.1.4 Classification 10
3.1.5 Operating conditions 10
3.1.6 Measurement specifications 11
3.1.7 Connectivity 11
3.2 TUBELESS BLOOD PRESSURE CUFF MODULES 12
4 QUICK MEASURING GUIDE 13
4.1 PREPARATION FOR MEASUREMENT 13
4.1.1 Pairing with MESI mTABLET UNIT 13
4.1.2 Assembly of the MESI TUBELESS CUFF UNIT 14
4.1.3 Patient preparation 14
4.1.4 Performing ABI measurement 16
4.2 RESULTS 18
5 DETAILED INSTRUCTIONS 18
5.1 FIRST TIME USE 18
5.1.1 Basic functionalities 18
5.1.2 AC/DC power supply and battery 19
5.1.3 Activation 19
5.1.4 First use Battery Status 19
5.1.5 Pairing 20
5.1.6 Attaching the cus 22
5.1.7 Detaching the cus 23

5.2 PATIENT SELECTION 24
5.2.1 Selecting the patient 24
5.2.2 Adding the patient 25
5.3 PERFORMING ABI MEASUREMENT 25
5.3.1 Cu placement 26
5.3.2 Performing the ABI measurement 30
5.4 REVIEWING ABI MEASUREMENT 32
5.4.1 MESI mTABLET result screen 33
5.5 INTERPRETATION OF ABI RESULT 34
5.5.1 Detection of severe PAD and incompressible arteries 34
5.5.2 Pulse waveform 34
5.5.3 Oscilation graph 36
5.6 MULTIFUNCTIONAL BUTTON 37
5.6.1 LED indicators 38
5.6.2 Button functions 38
6 MAINTENANCE 39
6.1 CHARGING THE BATTERY 39
6.2 CLEANING INSTRUCTIONS 39
6.3 DISINFECTION 40
6.4 PRODUCT LIFE AND STORAGE 41
7 GENERAL WARNINGS 42
7.1 PATIENT INJURIES PREVENTION 42
7.2 MEASUREMENT PROCEDURE 42
7.3 MAINTENANCE 44
7.4 FUNCTIONING OF THE DEVICE 44
8 ERRORS 45
9 TROUBLESHOOTING 47
10 WARRANTY INFORMATION 47
11 STANDARD COMPLIANCE 48
11.1 MANUFACTURER DECLARATION ON EMC 49
11.2 ESSENTIAL PERFORMANCE 56
12 IMPORTANT LABELS 56

All rights reserved. This publication may not be reproduced, copied
or stored on a memory device. Furthermore, this publication may
not be used for any purpose other than as the instructions for the
use of the MESI TUBELESS CUFF modules (part of MESI mTABLET
ABI). This publication may not be translated into other languages
or converted into other formats in any way without the prior written
permission of MESI Ltd.
1.2 SAFETY INFORMATION
The contents of the instructions for use may be altered without
notice. The latest version of the instructions for use is available at
www.mesimedical.com/support/mtablet/instructions-for-use.
To avoid personal injury and/or damaging the device or accessories,
follow the safety recommendations given below.
SAFETY
AND LEGAL
RECOMMEN-
DATIONS
1
SETUP AND
TECHNICAL
PERSONNEL
1.2.1
ACCESS TO
THE DEVICE
1.2.2
SAFETY
MEASURES
1.2.3
1.1 LEGAL INFORMATION
SAFETY AND LEGAL RECOMMENDATIONS
The device must be set up by authorised personnel with adequate
professional training and experience who are aware of all the
dangers in relation to the setup of the device and its use and who
will take adequate risk prevention measures for themselves, users,
other personnel and devices.
Only authorised persons may be given access.
Local safety requirements are to be complied with, if so, required
by regulations. Should there be any conflict between the safety
recommendations in this document and the recommendations
stipulated by local regulations, the local regulations take precedence.
The MESI mTABLET ABI users must be adequately trained to use the device. Before the first use
of the device, users must carefully read the entire instructions for use and follow the instructions
for use of the connected equipment.
7
PRODUCT DESCRIPTION
PRODUCT
DESCRIPTION
2
The MESI mTABLET ABI diagnostic system package includes the
following equipment:
• 4x Tubeless Blood Pressure Cu Module (including size M
cus for the left arm, right arm, left ankle and right ankle),
• a wireless medical tablet system (separate packaging), and
• a 4-port charging station module (separate packaging).
2.1 WHAT IS IN THE PACKAGE
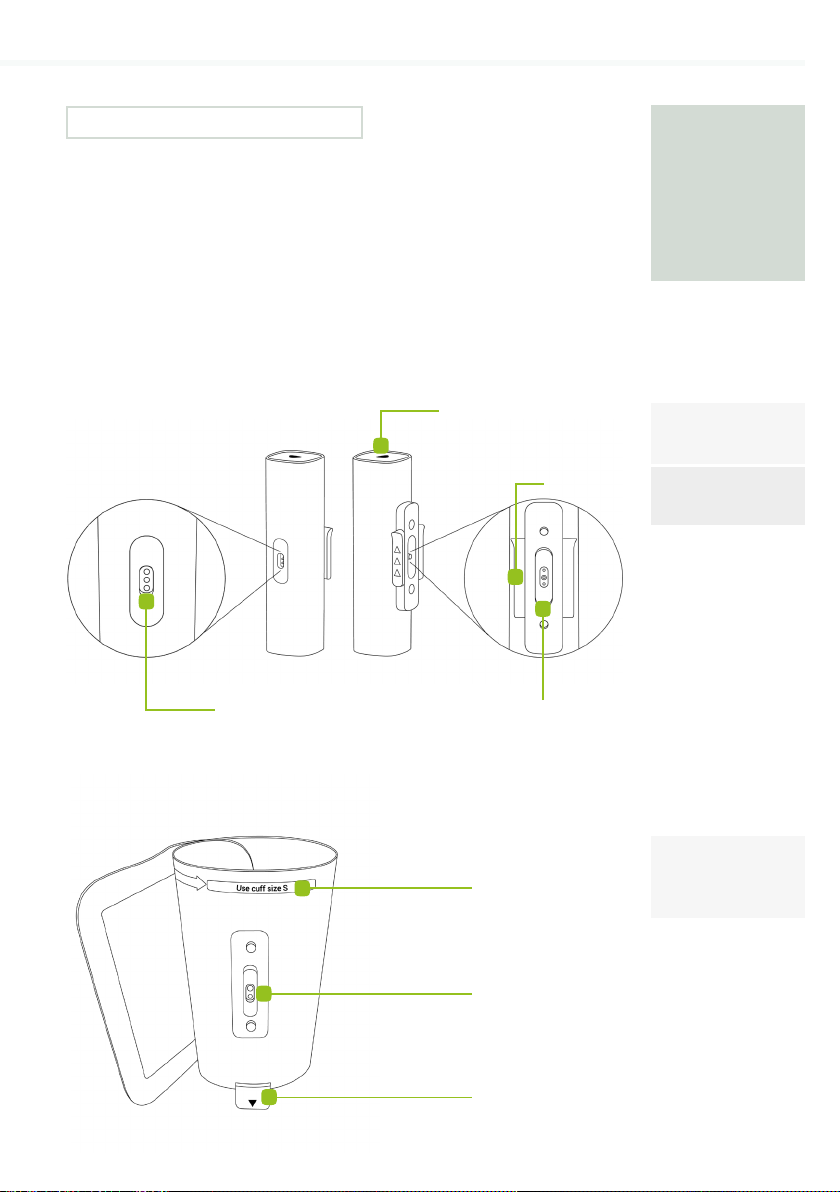
Model
CUFFMD
MESI tubeless
cu unit
Tubeless cu -
Medium
(LA, RA, LL, RL)
Multifunctional LED button
Sliding lock
Tubeless cu female
connector
Power connector
Size indicator
Tubeless cu male
connector
Medial ankle

8PRODUCT DESCRIPTION
The MESI mTABLET ABI is an automated wireless ankle-brachial
index measuring system for screening patients for Peripheral Arterial
Disease (PAD/LEAD). The system is intended to perform (and make
viewable and storable) adult ankle-brachial index (ABI) measurement.
It is a wireless system which is comprised of a wireless medical
tablet system, four tubeless blood pressure cu modules and a
4-port charging station module.
MESI mTABLET ABI is intended to be used solely in a professional
healthcare environment by trained healthcare personnel which
can correctly place blood pressure cus on a patient’s body, verify
that these cus are inflating or deflating normally and start the
measurement process.
MESI mTABLET ABI is intended to measure the Ankle-Brachial
Index by using a type of oscillometric method plethysmogra-
phy. The Ankle-Brachial Index result (along with blood pressure
values used in calculating the ABI), pulse wave and oscillation
graph are captured and displayed as a numerical and graphical
representation on the MESI mTABLET UNIT.
With standard software, the MESI mTABLET ABI supports an
automatic two-step measurement of systolic, diastolic and mean
arterial blood pressure on both upper arm and ankle locations.
The first part of the measurement performs a dual arm blood
pressure measurement while the second part of the measure-
ment performs a simultaneous ABI measurement considering
the higher of the two arm blood pressures and both ankle blood
pressures.
2.2 INTENDED USE
The MESI mTABLET ABI users must be adequately trained to use
device. Before the first use of the device, users must carefully read the
entire instructions for use and follow the Instructions for Use in full and
follow the Instructions for Use for connected equipment.
ACCESSORIES
2.1.1
9
PRODUCT DESCRIPTION
The device is recharged through the AC/DC power supply,
however, the MESI mTABLET ABI is not intended to be used
while connected to mains electricity.
10 TECHNICAL SPECIFICATIONS
DIMENSIONS
40 mm (1.57 inches)
Width
Depth 40 mm (1.57 inches)
150 mm (5.91 inches)
Height
Weight 286 g
POWER &
BATTERY
Rechargeable lithium-polymer battery
Battery type
Capacity 1240 mAh
> 200
Examinations per
battery charge
CLASSIFICATION
Class II equipment
Protection against
electric shock
Software
classification
Class B
3.1 MESI TUBELESS CUFF UNIT (CUFFMD)
3.1.1
3.1.2
3.1.4
CUFF SIZES
3.1.3 CUFF-RAM, CUFF-LAM, CUFF-RLM,
CUFF-LLM
Medium size cus
Circumference 22-32 cm
Large size cus CUFF-RAL, CUFF-LAL, CUFF-RLL,
CUFF-LLL
Circumference 32-43 cm
TECHNICAL
SPECIFICATI-
ONS
3
Listed below is technical information regarding the MESI TUBELESS
CUFF MODULE and its on-delivery specifications.
RF emissions
(CIPSR 11)
Group 1. Class B
OPERATING
CONDITIONS
10° to 40°CTemperature,
operating
Relative humidity 25 to 85% (no condensation)
3.1.5
Pressure during
operation
700 to 1060 hPa
IP42 Rating

11
TECHNICAL SPECIFICATIONS
The MESI mTABLET ABI users must be adequately trained to use the
device. Before the first use of the device, users must carefully read the
entire the Instructions for Use and follow the Instructions for Use for
connected equipment.
MEASUREMENT
SPECIFICATIONS
3.1.6
Measurement range:
• Pressure: 0 to 299mm Hg
• Heart rate: 30 to 199 beats per minute
Max deviation:
• Pressure: ± 3 mmHg
• Heart rate: ± 5% of value
• Ankle-Brachial Pressure Index: ± 0.1
CONNECTIVITY
3.1.6
Data connectivity with MTABMD (Bluetooth 2.1 + EDR)
Receiving section
Frequency range 2401.3 MHz – 2480.7 MHz
0.930 MHz
Bandwidth
Transmitter
Output power 0.5 - 4.5 dBm
2401.3 MHz – 2480.7 MHz
Frequency range
Modulation GFSK
Measurements using oscillometry and volume plethysmography:
• Ankle-Brachial Pressure Index
• Systolic blood pressure
• Diastolic blood pressure
• Heart rate

12 TECHNICAL SPECIFICATIONS
3.2 TUBELESS BLOOD PRESSURE CUFF MODULES
The MESI mTABLET ABI package includes 4 adult Tubeless Blood
Pressure Cu Modules (medium size) for automated wireless
ankle-brachial index measurement.
Provided tubeless cus:
• Right arm – red
• Left arm - yellow
• Right ankle – black
• Left ankle – green
NOTE
For more information regarding approved cus and other accessories see
instruction manual of the cu available in the MESI package or contact your
local distributor.

13
QUICK MEASURING GUIDE
QUICK
MEASURING
GUIDE
4
NOTE
Before using the device for the first time, read the Instructions for Use carefully
and follow the recommendations and suggestions. This Chapter only includes
short instructions for the use of the MESI mTABLET ABI. For detailed description
of short instructions on the device, see Chapter 5 DETAILED INSTRUCTIONS.
NOTE
When performing the Ankle-Brachial Index measurement the patient must be
in supine position and remain still.
NOTE
The MESI mTABLET ABI is intended for use in professional environment,
where measurements must be carried out by adequately trained medical
personnel. The MESI mTABLET ABI is not intended for home use.
NOTE
The MESI mTABLET ABI may be used on pregnant women.
NOTE
The MESI mTABLET ABI is not intended for use on new-borns or children
under the age of 10 years.
NOTE
In case of the presence of intravenous cannulas or arteriovenous (AV) fistulas,
the cus and measurement can cause injury to the limb.
4.1 PREPARATION FOR MEASUREMENT
MESI TUBELESS CUFF MODULES are a part of the MESI mTABLET ABI
system. Before starting a measurement be sure that you are familiar
with all devices and their instructions which are part of the system.
PAIRING WITH A
MESI mTABLET
UNIT
4.1.1
Before any measurements can be performed all MESI TUBELESS
CUFF UNIT devices need to be paired to the MESI mTABLET
UNIT. For detailed instructions please follow instructions in
Chapter 5.1.5 Pairing.

14 QUICK MEASURING GUIDE
During shipping and transportation, the conical cus are disconnected
from MESI TUBELESS CUFF UNITS. Before first use connect the
cus to the port on the MESI TUBELESS CUFF UNIT as shown on
the image below. Ensure that all cus are securely attached.
ASSEMBLY
OF THE MESI
TUBELESS
CUFF UNIT
4.1.2
PATIENT
PREPARATION
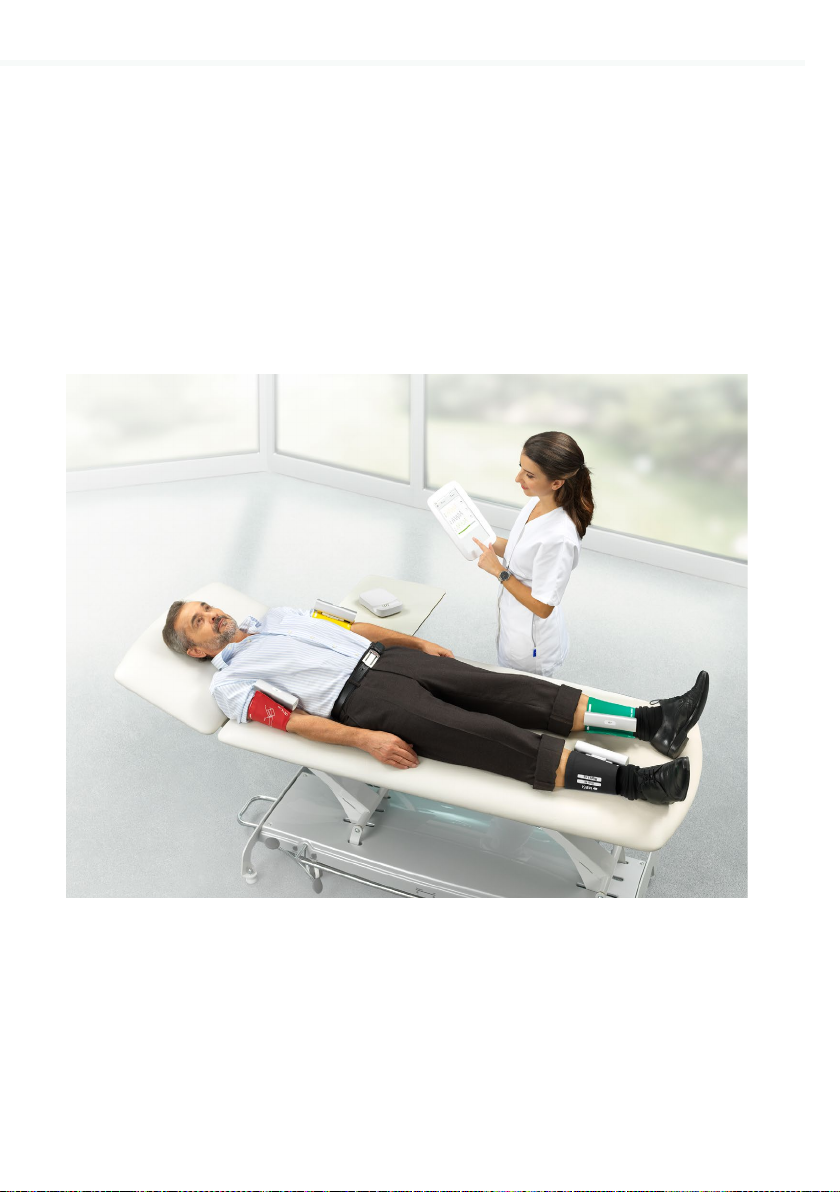
4.1.3 Patient needs to be in supine position, lie still and remain quiet.
Step 1 Choosing the right colour of the cu
Select the appropriate cu, depending on the description and the colour of the cu:
POSITION DESCRIPTION COLOUR
Right arm RIGHT ARM
On the cu Of the cu
RED
Left arm LEFT ARM YELLOW
Right ankle RIGHT ANKLE BLACK
Left ankle LEFT ANKLE GREEN

15
QUICK MEASURING GUIDE
NOTE
The cus can be connected to either of the MESI TUBELESS CUFF UNIT.
The colour, size and positioning will be automatically detected by the MESI
TUBELESS CUFF UNIT.
Step 2 Place the cus to the appropriate arm/leg
ARM:
• Position the cu 1-2 cm above the elbow joint. Align the label Artery with the Artery on inner
side of an arm.
• Place the cu so that there is two fingers’ width of room between the limb and the cu.
• Check that you have chosen the correct size using the INDEX marking and the OK area of
the cu.
ANKLE:
• Position the cu 2-3 cm above the ankle. Make sure that the label Ankle points towards
inner side of an ankle.
• Place the cu so that there is two fingers’ width of room between the limb and the cu.
Check that you have chosen the correct size using the INDEX marking and the OK area on
the cu.

16
PERFORMING ABI
MEASUREMENT
QUICK MEASURING GUIDE
4.1.4
On the MESI mTABLET UNIT select an existing
patient (1) or add a new one (2).
Step 1
1
2
NOTE
Observe instructions for use that are provided with the cu.
After selecting the patient, select the ABI
measurement in the application menu.
Step 2

17
QUICK RECORDING GUIDE
When starting the ABI application for
the first time the default settings can
be set by selecting between a 3-cu
or 4-cu ABI measurement (when
selecting a 3-cu measurement,
please select right or left arm) and
confirming.
These settings can be changed at
any time by pressing
Step 3
Observe the position indication on the cus and place them on appropriate arm or leg.
Then press START (1) and wait until the measurement is completed (2).
Step 4

18 QUICK MEASURING GUIDE/ DETAILED INSTRUCTIONS
4.2 RESULTS
Once the measurement has been taken the system
will automatically switch to the results page. By scrolling
on the results screen the ABI, oscillation graph, pulse
waveform and SmartArm™ selection can be reviewed.
On the top the navigation menu provides the following actions:
- retake the recording
- delete the recording
- share for second opinion
- print the result
NOTE
For more information about the result screen see Chapter 5.4 REVIEWING
ABI MEASUREMENTS
DETAILED
INSTRUCTIONS
5This Chapter contains all the information required by users of the
device for a safe, correct and accurate measurement. It includes a
detailed and complete description of all the functions of the device,
safety instructions and all the information required to understand
operation of the device.
5.1 FIRST TIME USE
BASIC
FUNCTIONALITIES
MESI mTABLET ABI package consists out of four MESI TUBELESS
CUFF UNIT diagnostic devices and 4 tubeless cus. Before first
use, the MESI TUBELESS CUFF UNITS need to be paired with MESI
mTABLET UNIT. Follow the instructions carefully.
5.1.1
19
DETAILED INSTRUCTIONS
ACTIVATION
When setting up the MESI TUBELESS CUFF UNIT for the first time
it needs to be activated. The device will not respond until it is
placed on the MESI LARGE CHARING PLATE and a multifunctional
button illuminates. It is recommended that before first use the MESI
TUBELESS CUFF UNIT is fully charged.
5.1.2
FIRST USE
BATTERY STATUS
The MESI TUBELESS CUFF UNIT comes with an internal battery. To
check the battery status, press multifunctional button on top of each
MESI TUBELESS CUFF UNIT. The button will respond by lighting
up in green or red depending on the battery state. Otherwise, see
the chapter 6.1 CHARGING THE BATTERY for more information on
battery charging.
5.1.3
NOTE
The battery inside a completely new device is most likely not completely
empty and can provide enough power to start the device up. Nonetheless,
please recharge the batteries before first use.
NOTE
When the battery needs to be replaced, the MESI mTABLET UNIT will display
a battery warning. For more information see Chapter 8 ERRORS.
AC/DC POWER
SUPPLY AND
BATTERY
The MESI TUBELESS CUFF UNIT uses two power ...sources: mains
electricity (which uses a AC/DC power supply for charging) and
battery power (which is used while performing measurements).
Connect the AC/DC power supply to a wall socket with a mains
voltage of 100-240V at 50-60Hz and to the connector at the back
of the device.
5.1.4
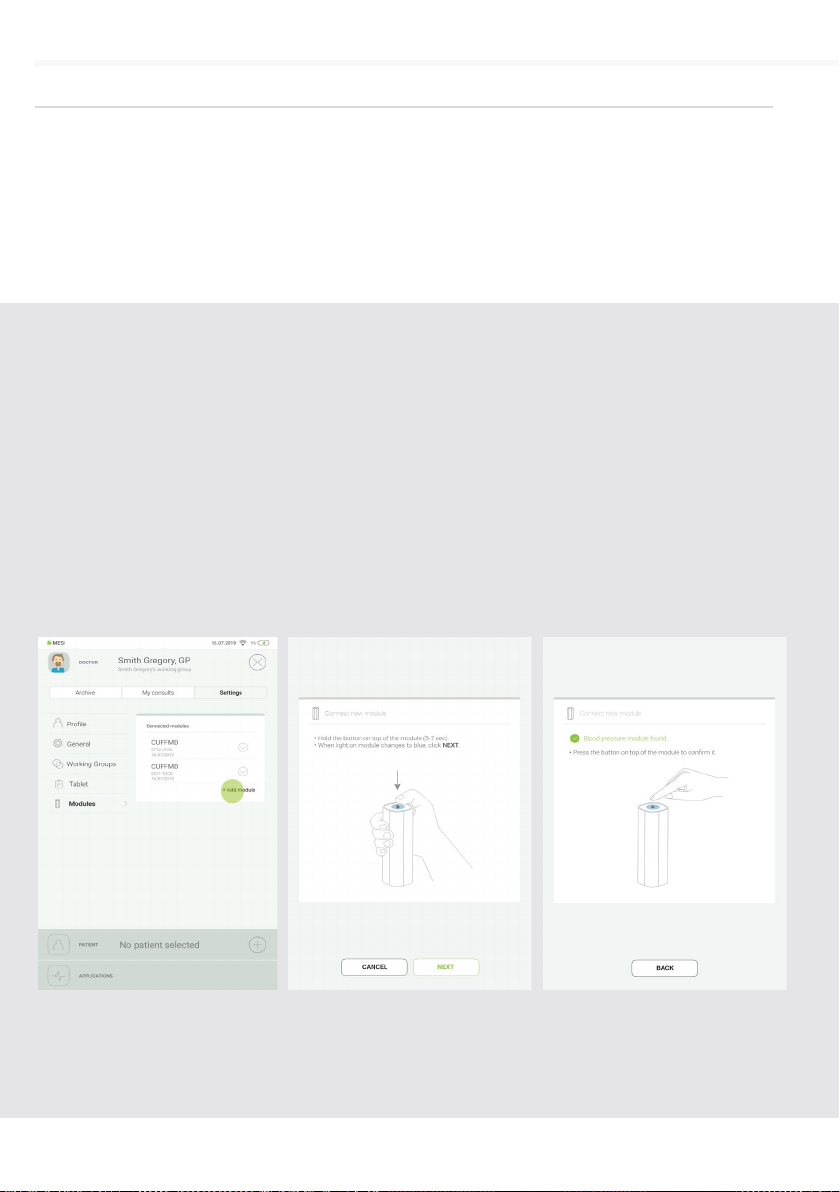
20 DETAILED INSTRUCTIONS
Go to User profile > Settings
> Modules > +Add module
Step 1
Press and hold the button
on the top of the MESI
TUBELESS CUFF UNIT until
the multifunctional button light
changes to blue.
Step 2
When the MESI mTABLET UNIT
establishes a connection with
MESI TUBELESS CUFF UNIT,
the light on top of the module
will change to green. Confirm
the pairing process by pressing
the button on top of the
module again.
Step 3
PAIRING
5.1.5 Before any measurements can be performed the MESI TUBELESS
CUFF UNIT has to be paired with the MESI mTABLET UNIT. Take
the MESI mTABLET UNIT, open user profile (for more information
about user accounts see MESI mTABLET instruction manual, chapter
MANAGING USERS) and observe the following instructions.
Table of contents
Other MESI Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Active Medical
Active Medical 77644 Device instructions

Arthrex
Arthrex Lift-Assist AR-1627 Instructions for use

ResMed
ResMed Lumis 100 VPAP S user guide

Thoratec
Thoratec PediMag Instructions for use
Huntleigh
Huntleigh D920 Instructions for use

LionsGate Technologies
LionsGate Technologies Kenek Edge user manual