MESI mTABLET TBI User manual

Instructions
for Use
MESI mTABLET TBI
Toe-Brachial Index
ISO 9001 Q-1664
ISO 13485 M-049
MTABSYSTBI

CONTACT INFORMATION
Address MESI, development of medical devices, Ltd
Leskoškova cesta 11a
SI-1000 Ljubljana
Slovenia, European Union
Telephone +386 (0)1 620 34 87
E-mail [email protected]
Website www.mesimedical.com
DISTRIBUTOR INFORMATION

Instructions
for Use
ISO 9001 Q-1664
ISO 13485 M-049
MESI mTABLET TBI
Toe-Brachial Index

4
1 SAFETY AND LEGAL RECOMMENDATIONS 7
1.1 LEGAL INFORMATION 7
1.2 SAFETY INFORMATION 7
1.2.1 Setup and Technical Personnel 7
1.2.2 Access to the Device 7
1.2.3 Safety Measures 7
2 PRODUCT DESCRIPTION 8
2.1 WHAT IS IN THE PACKAGE? 8
2.1.1 Accessories 9
2.2 INTENDED USE 9
3 TECHNICAL SPECIFICATIONS 11
3.1 MESI TOE BLOOD PRESSURE UNIT (TBPMD) 11
3.1.1 Dimensions 11
3.1.2 Power & Battery 11
3.1.3 Cu Sizes – Toe 11
3.2 MESI TUBELESS CUFF UNIT (CUFFMD) 11
3.2.1 Dimensions 11
3.2.2 Power & Battery 12
3.2.3 Cu sizes – Arm 12
3.3 DEVICE SPECIFICATIONS 12
3.3.1 Classification 12
3.3.2 Operating Conditions 12
3.3.3 Measurement Specifications 13
3.3.4 Connectivity 13
4 QUICK MEASURING GUIDE 14
4.1 PREPARATION FOR MEASUREMENT 14
4.1.1 Pairing with the MESI mTABLET UNIT 14
4.1.2 Assembly of the MESI TOE BLOOD PRESSURE UNIT 15
4.1.3 Assembly of the MESI TUBELESS CUFF UNIT 15
4.1.4 Patient Preparation 16
4.1.5 Performing a TBI Measurement 17
4.2 RESULTS 20
5 DETAILED INSTRUCTIONS 20
5.1 FIRST TIME USE 20
5.1.1 Basic Functionalities 20

5
5.1.2 AC/DC Power Supply and Battery 21
5.1.3 Activation 21
5.1.4 First Use Battery Status 21
5.1.5 Pairing 22
5.1.6 Attaching the Toe Blood Pressure Cable to the Unit 24
5.1.7 Attaching the Arm Cus to the Unit 25
5.1.8 Detaching the Arm Cus to the Unit 26
5.2 PATIENT SELECTION 27
5.2.1 Selecting a Patient 27
5.2.2 Adding a Patient 28
5.3 PERFORMING A TBI MEASUREMENT 28
5.3.1 Cu Placement 29
5.3.2 Performing a TBI Measurement 33
5.4 REVIEWING A TBI MEASUREMENT 36
5.4.1 MESI mTABLET Results Screen 37
5.4.1.1 Navigation Area 37
5.4.1.2 Measurement Information 37
5.4.1.3 PPG Pulse Waveform Recordings 37
5.4.1.4 Oscillation Graphs and Pulse Waveform Recordings 37
5.4.1.5 Patient Measurement History 37
5.4.1.6 Comments Area 38
5.5 INTERPRETATION OF A TBI RESULT 38
5.5.1 Detection of Systolic Blood Pressure in Toes 38
5.5.2 PPG Pulse Waveforms Interpretation and Toe Blood Pressure 38
5.6 MULTIFUNCTIONAL BUTTON 40
5.6.1 LED Indicators 40
5.6.1.1 Standby 41
5.6.1.2 Charging 41
5.6.1.3 Pairing 41
5.6.2 Button Functions 41
5.6.2.1 Standby 41
5.6.2.2 Measurement Mode 41
6 MAINTENANCE 42
6.1 CHARGING THE BATTERY 42
6.2 CLEANING INSTRUCTIONS 42
6.3 DISINFECTION 44
6.4 PRODUCT LIFE AND STORAGE 45
7 GENERAL WARNINGS 45
7.1 PATIENT INJURY PREVENTION 45
7.2 MEASUREMENT PROCEDURE 46

7.3 MAINTENANCE 47
7.4 DEVICE FUNCTIONALITY 47
8 ERRORS 48
9 TROUBLESHOOTING 50
10 WARRANTY INFORMATION 50
11 STANDARD COMPLIANCE 51
11.1 MANUFACTURER DECLARATION ON EMC 52
11.2 ESSENTIAL PERFORMANCE 57
12 IMPORTANT LABELS 57
NOTES 58

The MESI mTABLET TBI users must be adequately trained in use of the
device. Users must carefully read the entire Instructions for Use prior
to initial use of the device and follow the Instructions for Use for the
connected equipment.
All rights reserved. This publication may not be reproduced, copied
or stored on a memory device. Furthermore, this publication may
not be used for any purpose other than as the instructions for the
use of the MESI TOE BLOOD PRESSURE UNIT and MESI TUBELESS
CUFF UNITS (part of the MESI mTABLET TBI). This publication may
not be translated into other languages or converted into other
formats in any way without the prior written permission of MESI Ltd.
The contents of these Instructions for Use may be altered without
notice. The latest version of the Instructions for Use is available at
www.mesimedical.com/support/mtablet/instructions-for-use.
SAFETY
AND LEGAL
RECOMMEN-
DATIONS
1.2 SAFETY INFORMATION
SETUP AND
TECHNICAL
PERSONNEL
SAFETY
MEASURES
ACCESS TO
THE DEVICE
To avoid personal injury and/or damaging the device or accessories,
follow the safety recommendations given below.
The device must be set up by authorised personnel with adequate
professional training and experience who are aware of all the
dangers in relation to the setup of the device and its use and who
will take adequate risk prevention measures for themselves, users,
other personnel and devices.
Only authorised persons may be given access.
Local safety requirements are to be complied with, if so required
by regulations. In addition to local safety regulations the safety
instructions in this document shall also to be complied with. Should
there be any conflict between the safety recommendations in this
document and the recommendations stipulated by local regulations,
the local regulations take precedence.
1
1.2.1
1.2.2
1.2.3
1.1 LEGAL INFORMATION
SAFETY AND LEGAL RECOMMENDATIONS 7

8PRODUCT DESCRIPTION
PRODUCT
DESCRIPTION
2
The MESI TBI MODULE is a wireless Toe-Brachial Index module
designed for the MESI mTABLET TBI System. Toe-Brachial Index
measurements and other parameters are displayed on the MESI
mTABLET.
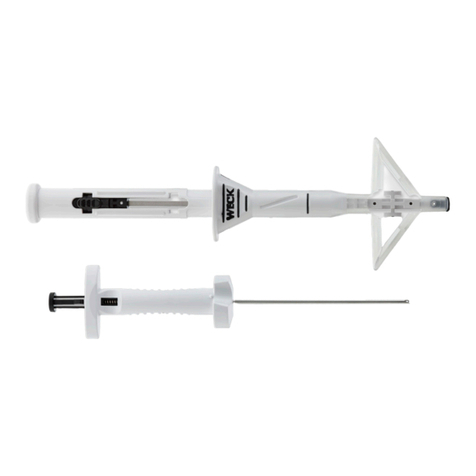
The MESI TBI MODULE package includes the following
equipment:
• Toe Blood Pressure Unit (TBPMD),
• 1x Toe Blood Pressure cable,
• Digit cus pair - Medium (ML, MR),
• Digit cus pair - Large (LL, LR),
• 2x Tubeless Cu Unit (CUFFMD),
• 2x Tubeless Cu - Medium (RA, LA),
• 120x pre-cut medical tape,
• 2x fastener strap,
• A wireless medical tablet system (separate packaging),
• A 4-port charging station module (separate packaging),
• Instructions for Use,
• Calibration Report, and
• Declaration of Conformity.
2.1 WHAT IS IN THE PACKAGE?

9
PRODUCT DESCRIPTION
The MESI mTABLET TBI is an automated wireless Toe-Bra-
chial Index measuring system for screening patients for
Peripheral Arterial Disease (PAD/LEAD). The system is in-
tended to perform, view and store Toe-Brachial Index (TBI)
measurements of adult patients - patients in the PAD risk
group - especially diabetic, renal, and elder patients who
may suer from calcified vessels. It is a wireless system
which is comprised of a wireless medical tablet system, toe
blood pressure module, two tubeless blood pressure cu
modules and a 4-port charging station module.
The MESI mTABLET TBI is intended to be used in a profes-
sional clinical environment by trained healthcare person-
nels who understand the principle of Toe-Brachial Index
(TBI) measurement and are able to place arm cus, digit
cus and PPG probes on the patient body, as well as being
able to verify that these accessories are working as intend-
ed, and to start the measurement process.
The MESI mTABLET TBI is intended to measure Toe-Brachi-
al Index by performing non-invasive, plethysmography-os-
cillometric measurements of systolic brachial blood pres-
sures and photoplethysmographic (PPG) measurements of
systolic toe blood pressures. The Toe-Brachial Index result
2.2 INTENDED USE
ACCESSORIES
2.1.1
NOTE
Contact your local distributor for more information about dierent cu sizes
and other accessories.
MESI mTABLET TBI users must be adequately trained in use of the
device. Users must carefully read and follow the entire Instructions for
Use prior to initial use of the device.
Only use accessories and other parts recommended or supplied by
MESI. Use of parts not recommended or supplied by MESI may result
in injury, inaccurate information and/or damage to the unit. Follow the
instructions that are provided with the specific accessory.

10 PRODUCT DESCRIPTION
(along with used blood pressure values used in calculating
the TBI), PPG waveforms, pulse wave and oscillation graph
are captured and displayed as a numerical and graphical
representation on the MESI mTABLET UNIT.
The MESI mTABLET TBI supports automatic, one-step
measurements of systolic and diastolic brachial pressures
and systolic blood pressures in big toes using standard
software.
The device is recharged through an AC/DC power supply,
however, the MESI mTABLET TBI is not intended to be
used while connected to mains electricity.

11
The technical information regarding the MESI TOE BLOOD PRESSURE
UNIT and the MESI TUBELESS CUFF UNIT provided within the
package is as follows:
TECHNICAL SPECIFICATIONS
TECHNICAL
SPECIFICA-
TIONS
3
3.1 MESI TOE BLOOD PRESSURE UNIT (TBPMD)
DIMENSIONS
DIMENSIONS
40 mm (1.57 inches)
Width
Depth 40 mm (1.57 inches)
150 mm (5.91 inches)Height
Weight 244 g
40 mm (1.57 inches)
Width
Depth 40 mm (1.57 inches)
150 mm (5.91 inches)Height
Weight 286 g
3.1.1
3.2.1
POWER &
BATTERY
3.1.2
Rechargeable lithium-polymer battery
(LP602248)
Battery Type
Capacity 1240 mAh
FW8030M/05 (FRIWO FOX30-XM)AC/DC adaptor
CUFF SIZES TOE
3.1.3
Input 100-240V AC/50-60Hz/600-300mA
Output
Examinations per
Battery Charge
Charge Time for
Depleted Battery
5V DC/5.0A
> 200
approximately 2 hours
3.2 MESI TUBELESS CUFF UNIT (CUFFMD)
Medium-sized Cus
Dimensions
Large-sized Cus
Dimensions
Digit cus pair - Medium
90 x 20 mm
Digit cus pair - Large
120 x 25 mm

12 TECHNICAL SPECIFICATIONS
POWER &
BATTERY
3.2.2 Rechargeable lithium-polymer battery
(LP602248)
Battery Type
Capacity 1240 mAh
FW8030M/05 (FRIWO FOX30-XM)AC/DC Adaptor
CUFF SIZES
ARM
3.2.3
Input 100-240V AC/50-60Hz/600-300mA
Output
Examinations per
Battery Charge
5V DC/5.0A
> 200
3.3 DEVICE SPECIFICATIONS
Medium-sized Cus
Circumference
Large-sized Cus
Circumference
Tubeless cu set - BP - Medium
22-32 cm
Tubeless cu set - BP - Large
32-43 cm
CLASSIFICATION
3.3.1
OPERATING
CONDITIONS
3.3.2
Class II
Protection against
Electric Shock
Medical Device
Classification
Class IIa
Applied Parts
Software
Classification
RF Emissions
(CIPSR 11)
Type BF applied part
Class B
Group 1. Class A
10° to 40°COperating
Temperature
Relative Humidity
Ingress Protection
Rating
25 to 85% (no condensation)
IP42
Pressure during
Operation
700 to 1060 hPa
If the device is used or stored outside the specified environmental
parameters, the accuracy specified within the technical specifications
of the device is not guaranteed.

13
TECHNICAL SPECIFICATIONS
MEASUREMENT
SPECIFICATIONS
3.3.3
Measurement Range:
• Arm pressure: 0 to 299 mmHg
• Toe pressure: 20 to 250 mmHg
• Heart rate: 30 to 199 beats per minute
Max Deviation:
• Pressure: ± 3 mmHg
• Heart rate: ± 5% of value
• Toe-Brachial Pressure Index: ± 0.1
Measurements Using Oscillometry, Volume Plethysmography
and Photoplethysmography:
• Toe-Brachial Pressure Index
• Systolic blood pressure (arms and toes)
• Diastolic blood pressure (arms)
• Heart rate
CONNECTIVITY
3.3.4
Data connectivity with MTABMD (Bluetooth 2.1 + EDR)
Receiving Section
Frequency Range 2401.3 MHz – 2480.7 MHz
0.930 MHz
Bandwidth
Transmitter
Output Power 0.5 - 4.5 dBm
2401.3 MHz – 2480.7 MHz
Frequency Range
Modulation GFSK
Temperature sensors for measuring skin temperature are integrated
into both PPG probes. Skin temperature can vary significantly from
core body temperature and is shown as additional information.
14
QUICK
MEASURING
GUIDE
4NOTE
NOTE
NOTE
NOTE
NOTE
Before using the device for the first time, read the Instructions for Use carefully
and follow the recommendations and suggestions. This chapter only includes
short instructions for the use of the MESI mTABLET TBI. See Chapter 5
DETAILED INSTRUCTIONS for a detailed description of the device's individual
functions.
When performing TBI measurements patients must be in a supine position
and remain still.
The MESI mTABLET TBI may be used on pregnant women.
The MESI mTABLET TBI is not intended for use on new-borns or children
under 10 years of age.
If intravenous cannulas or arteriovenous (AV) fistulas are present, cus and
measurements can cause injury to the limb.
4.1 PREPARATION FOR MEASUREMENT
PAIRING WITH THE
MESI mTABLET
UNIT
4.1.1 Before any measurements can be performed MESI TOE BLOOD
PRESSURE UNIT and MESI TUBELESS CUFF UNIT devices need
to be paired to the MESI mTABLET UNIT. For detailed instructions
please follow the instructions in Chapter 5.1.5 Pairing.
QUICK MEASURING GUIDE
MESI mTABLET TBI users must be adequately trained in use of the
device. Users must carefully read and follow the entire Instructions for
Use prior to initial use of the device.

15
QUICK MEASURING GUIDE
ASSEMBLY
OF THE MESI
TOE BLOOD
PRESSURE UNIT
ASSEMBLY
OF THE MESI
TUBELESS CUFF
UNIT
4.1.2
4.1.3
The MESI Toe Blood Pressure cable should be disconnected
during storage and transportation. Prior to initial use connect the
toe blood pressure cable to the port on the MESI TOE BLOOD
PRESSURE UNIT, as shown on the image below.
After ensuring that the cable is securely connected, attach the
digit cus into corresponding cu connector as shown on the
image below. Please ensure that the cu colours match the
pressure hose colours.
During shipping and transportation, the conical cus should be
disconnected from the MESI TUBELESS CUFF UNITS. Prior to
initial use connect the cus to the port on the MESI TUBELESS
CUFF UNIT as shown on the image below. Ensure that all cus
are securely attached.
Only use accessories and other parts recommended or supplied
by MESI. Use of accessories and other parts not recommended or
supplied by MESI may result in injury, inaccurate information and/or
damage to the unit. This product is not designed for sterile use.

16 QUICK MEASURING GUIDE
PATIENT
PREPARATION
4.1.4 The patient must be in supine position and remain still and quiet.
Step 1 Choosing the right colour of the cu
Step 2 Place the cus to the appropriate arm
Step 4 Place the cus on big toes
Step 3 Place the digit cus and photoplethysmographic probes to the appropriate big toe
Select the appropriate cu, depending on the description and the colour of the cu:
Arm:
Toe:
• Place the appropriate cu on left/right arm and position the cu 1-2 cm above the elbow joint.
• Make sure that the arrow-shaped artery marking is in line with the brachial artery.
• Place the appropriate digit cu on the selected toe and wrap it around the base of it. Make sure
the cus are wrapped around the toes tightly but comfortably.
• Place the PPG probe against the skin on the fleshy part of the appropriate big toe and secure it
with the enclosed fastener strap or medical tape.
• Make sure that the vertical indicator line falls within area marked with the OK sign. If not, select
an appropriately sized cu.
POSITION DESCRIPTION COLOUR
Right Arm RIGHT ARM
On the cu Of the cu
RED
Left Arm LEFT ARM YELLOW
Right Toe RIGHT TOE BLACK
Left Toe LEFT TOE GREEN
NOTE
The arm cus can be connected to either of the MESI TUBELESS CUFF
UNITS. The colour, size and positioning will be automatically detected by the
MESI TUBELESS CUFF UNIT.

17
PERFORMING
A TBI
MEASUREMENT
4.1.5
On the MESI mTABLET UNIT select existing
patient (1) or add a new one (2).
Step 1
After selecting the patient, please select the
TBI measurement in the application menu.
Step 2
NOTE
For additional information see the MESI mTABLET Instruction Manual –
Chapter 8.1 PATIENT SELECTION.
1
2
QUICK MEASURING GUIDE

18
When starting the TBI applica-
tion for the first time the default
settings can be set by selecting
between a 3-cu or 4-cu (1) TBI
measurement (when selecting a
3-cu measurement, please se-
lect right or left arm) and confirm
(2).
These settings can be changed at
any time by pressing .
Step 3
Observe the position indication on the cus and place them on the appropriate arm.
Step 4
Options
Select type of measurement:
4-cuff SmartArm 3-cuff arm selection
Select default arm to measure TBI:
Left arm Right arm
Display skin temperature during measurement:
Yes No
At 3-cuff manual measurement provider chooses an arm to
measure TBI. Recommended is to use an arm with higher blood
pressure.
Save as default
Reset to factory settings2
OK
CANCEL
TM
Options
Select type of measurement:
4-cuff SmartArm 3-cuff arm selection
Select default arm to measure TBI:
Left arm Right arm
Display skin temperature during measurement:
Yes No
At 3-cuff manual measurement provider chooses an arm to
measure TBI. Recommended is to use an arm with higher blood
pressure.
Save as default
Reset to factory settings2
OK
CANCEL
TM
QUICK MEASURING GUIDE
1 2

19
Wrap the digit cus around the base of the appropriate big toe. Make sure that the cus are not too
tight to prevent any residual pressure. Place the PPG probe against the skin on the fleshy part of the
appropriate big toe and secure it with the enclosed fastener strap or medical tape. Afterwards press
CONTINUE (1). Wait for PPG waveforms to stabilise. Once the waveforms are stabilised, press START
(2) and wait until measurement is completed.
Step 5
If required use the calliper tool (1) by sliding the graph
sideways to adjust the pressure reading. Afterwards press
CONFIRM (2). By pressing the SKIP button (3), the result will
be calculated with automatically detected pressures.
Step 6
QUICK MEASURING GUIDE
12
1
2
3
20 QUICK MEASURING GUIDE/DETAILED INSTRUCTIONS
4.2 RESULTS
Once the measurement has been taken the system will automatically
switch to the results screen. By scrolling on the results page additional
TBI measurement parameters can be viewed. The following actions
are listed on the top the navigation menu:
- retake the measurement
- delete the measurement
- share for second opinion
- print the result
- Go to: previous/next result
NOTE
For more information about the results screen see Chapter 5.4 REVIEWING
TBI MEASUREMENT.
DETAILED
INSTRUCTIONS
5This Chapter contains all the information required by users of the
device for safe, correct and accurate measurements. It includes a
detailed and complete description of all the functions of the device,
safety instructions and all the information required to understand
operation of the device.
5.1 FIRST-TIME USE
BASIC
FUNCTIONALITIES
MTABSYSTBI is a wireless system intended for measuring Toe-Brachial
Index (TBI). System is comprised of MESI mTABLET UNIT, MESI TOE
BLOOD PRESSURE UNIT, 2x MESI TUBELESS CUFF UNIT and MESI
LARGE CHARGING PLATE (CS4SYS).
5.1.1
This manual suits for next models
1
Table of contents
Other MESI Medical Equipment manuals