2/3
3. Material
Board: Thermosetting resin laminated board
Insertion shaft A: Stainless steel rod
Insertion shaft B: Stainless steel rod
Velcro fastening: Nylon
Unlock button: Polyacetal
⑪⑫ Bracket: Stainless steel rod
Fixing handle: Stainless steel rod
Cassette board: Thermosetting resin laminated board
Radiographic top leg: Polycarbonate
Pad: Urethane foam
Note: The numbers match the ones that are used in 1. Shape, Fig. 1-4.
4. Maximum patient weight
135 kg
Intended purpose
This is an accessory which is supplied with the operating table. The
adapter makes it possible to attach the back plate side mounting
accessories onto the leg plate sides by attaching them onto the
waist plate with the leg plates detached.
Intended user
This product is to be used by health care professionals, including
but not limited to surgeons, nurses and biomedical technicians.
Instructions for use
1. 08-077-70/-60/-60-R1/-60-R2
Insertion shaft Radiographic top
Pad Unlock button
1-1. Insert the Leg-side Reversal Adapter insertion shaft into
the leg plate insertion hole of the operating table.
1-2. Pull on the Leg-side Reversal Adapter to make sure it is
firmly inserted.
1-3. Attach the pad .
1-4. If radiography is required, attach the radiographic top
between the Leg-side Reversal Adapter and pad .
1-5. When detaching the Leg-side Reversal Adapter, then pull
the Leg-side Reversal Adapter straight out while pressing
the unlock button ④.
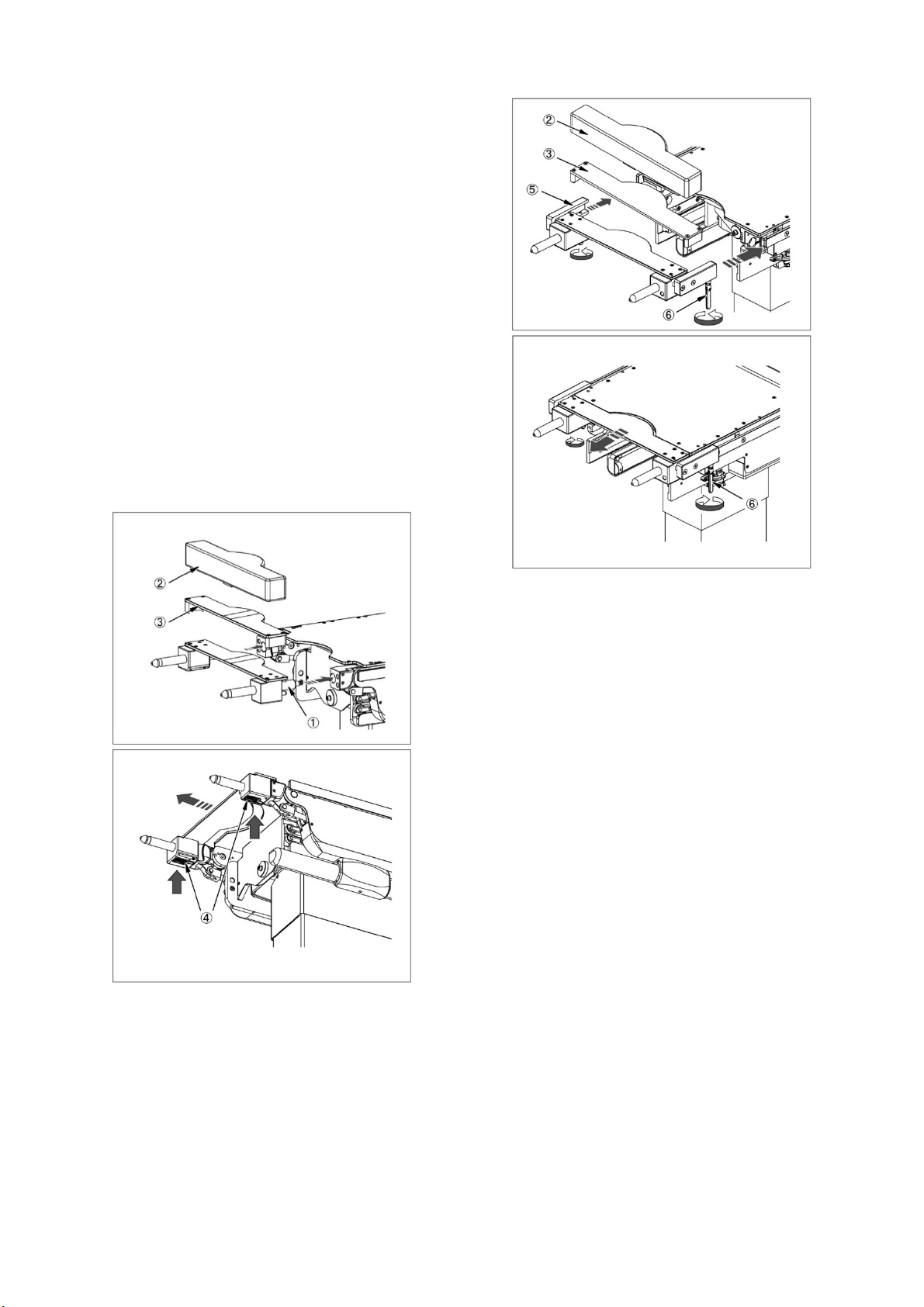
2. 08-077-50/-50-R1/-50-R2
Pad Bracket
Radiographic top Fixing handle
2-1. Insert the bracket of the Leg-side Reversal Adapter into
the side rails of the seat board on the operating table.
2-2. Make sure that the Leg-side Reversal Adapter is completely
inserted, and then tighten the fixing handle to fix it.
2-3. Attach the pad .
2-4. If radiography is required, attach the radiographic top
between the Leg-side Reversal Adapter and pad .
2-5. When detaching the Leg-side Reversal Adapter, then loosen
the fixing handle ⑥, and pull the Leg-side Reversal Adapter
straight out.
3. Applicable operating tables
Mizuho operating tables
Warning / Caution
1. Important caution
1-1. For hygiene, be sure to use sterilized drapes on the areas on
this product where the patient comes into contact with it.
1-2. Ensure that all of the fastener components are tied up. Failure
to do so may result in a patient injury.
1-3. When working with an operating table, take care not to allow
this product to make contact or interfere with the table top or
with other tools and appliances used in combination with this
product.
2. Interaction
[Precautions when using with other items]
If the operating table’s slide is slid the maximum amount with this
product and accessories attached to it, then the operating table’s
operations might be slowed down.
Storage / Life
1. Do not store the device in areas where there are high
temperatures or high humidities, nor in places where there are
drastic temperature or humidity fluctuations.
2. Service life of this product: 7 years
(When it is maintained and inspected as specified, and when it
is properly stored.)
Fig. 5
Leg-side Reversal Adapter A
Fig. 6
Leg-side Reversal Adapter A
Fig. 7
Fig. 8


