ShenZhen Luckcome Technology Inc., Ltd OCT BABY iFM-10B Series User manual

OCT BABY
iFM-10B Series Fetal Doppler
User Manual
Version No.: V1.0
Date: 2015. 03
File No.: 503-020048-011

iFM-10B Series Fetal Doppler User Manual
1
Table of Content
1 Safety Guide.................................................................................... 2
2 Product Introduction.......................................................................4
2.1 General Information.............................................................4
2.2 Features................................................................................4
2.3Configurations.......................................................................4
3 Transducer and software.................................................................5
3.1 Transducer............................................................................5
3.2 Software............................................................................... 6
4 Device Operations.........................................................................14
4.1FHR Monitoring...................................................................14
4.2 Fetal Heart Positioning.......................................................15
4.3 Fetal Movement................................................................. 16
4.4Record................................................................................. 16
4.5 Power recharge.................................................................. 18
5 Technical Data............................................................................... 18
6 Maintenance................................................................................. 21
7 Warranty....................................................................................... 23
8 Storage and Transportation.......................................................... 24
Tips for Fetal Monitoring..................................................................25

iFM-10B Series Fetal Doppler User Manual
2
1 Safety Guide
This device is internally powered equipment, and the degree of
protection against electric shock is Type B applied part. It is IPX1
transducer.
Before use it, please check its lifetime. Its lifetime is 5 years and
manufacturing date is labeled at the bottom side of device.
1.1 Important Notice
To avoid the potential harm, please make sure operating
complies with follows:
Warning: It is not suitable for using in the environment with
flammable gas, such as anesthesia gas, to avoid exploding.
Warning: Do not put the battery into the fire, it will cause
exploding.
Warning: Use on one patient only at the same time.
Warning: It is not against defibrillation devices. Do not use it if
there is high frequency surgical unit operating, MRI
checking. It will do harm to operator and patient.

iFM-10B Series Fetal Doppler User Manual
3
Warning: The efficient working distance is 5 meters.
Caution: This instrument must be maintained by qualified
engineers.
Caution: This instrument is designed to work continuously, and
water drop proof type, and be care to avoid to be
splashed.
Caution: Avoid vibrating; keep it clean
Caution: Do not sterilize it in high-temperature environment, or use
electronic beam or γ beam.
Caution: Before using the instrument, please check if there is any
damage of equipment that may affect the patient’s safety
or the instrument performance. The recommended check
period is one month or shorter. If an obvious damage is
found, it should be solved before use.
Caution: Following safety check needs to be done by qualified,
experienced personnel about every 2 years or follow local

iFM-10B Series Fetal Doppler User Manual
4
government regulations:
Check if there is mechanical or functional damage
Check if safety labels are clear
Check its function works in compliance with user
manual.
Caution: When the device is out of its lifetime, follow local laws to
return to manufacturer for recycling.
Caution: The battery should get properly treatment according to
local rules after the capability of battery run out.
Caution: This instrument is a hand-held FHR checking device; it
can’t replace the standard fetal monitoring. End user needs
to get professional personals guidance before 1st time
using.
Caution: We recommend using minimum monitoring time after
meeting clinical monitoring need.
Caution: Do not use the device before battery is well installed.

iFM-10B Series Fetal Doppler User Manual
5
2 Product Introduction
2.1 General Information
iFM-10B series Fetal Doppler is hand-held monitoring devices. It
is made of main unit including battery, transducer and APP
software. It is applicable for monitoring of fetal heart rate.
Model No.
Connection between transducer and mobile
phone
iFM-10B
Bluetooth
2.2 Features
Use same DSP technology as fetal monitors to monitor fetal
heart rate, ensure its accuracy and reliability.
Very sensitive transducer, the ultrasound power is far lower
than Industrial standard, is safe to the fetus.
User-friendly interface, easy to use.

iFM-10B Series Fetal Doppler User Manual
6
Fetus Heart Sound output through mobile phone
App software features with review, share and antenatal
training music.
2.3Configurations
USB cable ……………………………………………………1piece
Audio cable…………………………………………………1piece
Ultrasound transducer…………………………………1 piece
User Manual, Warranty Card……………………….. 1set
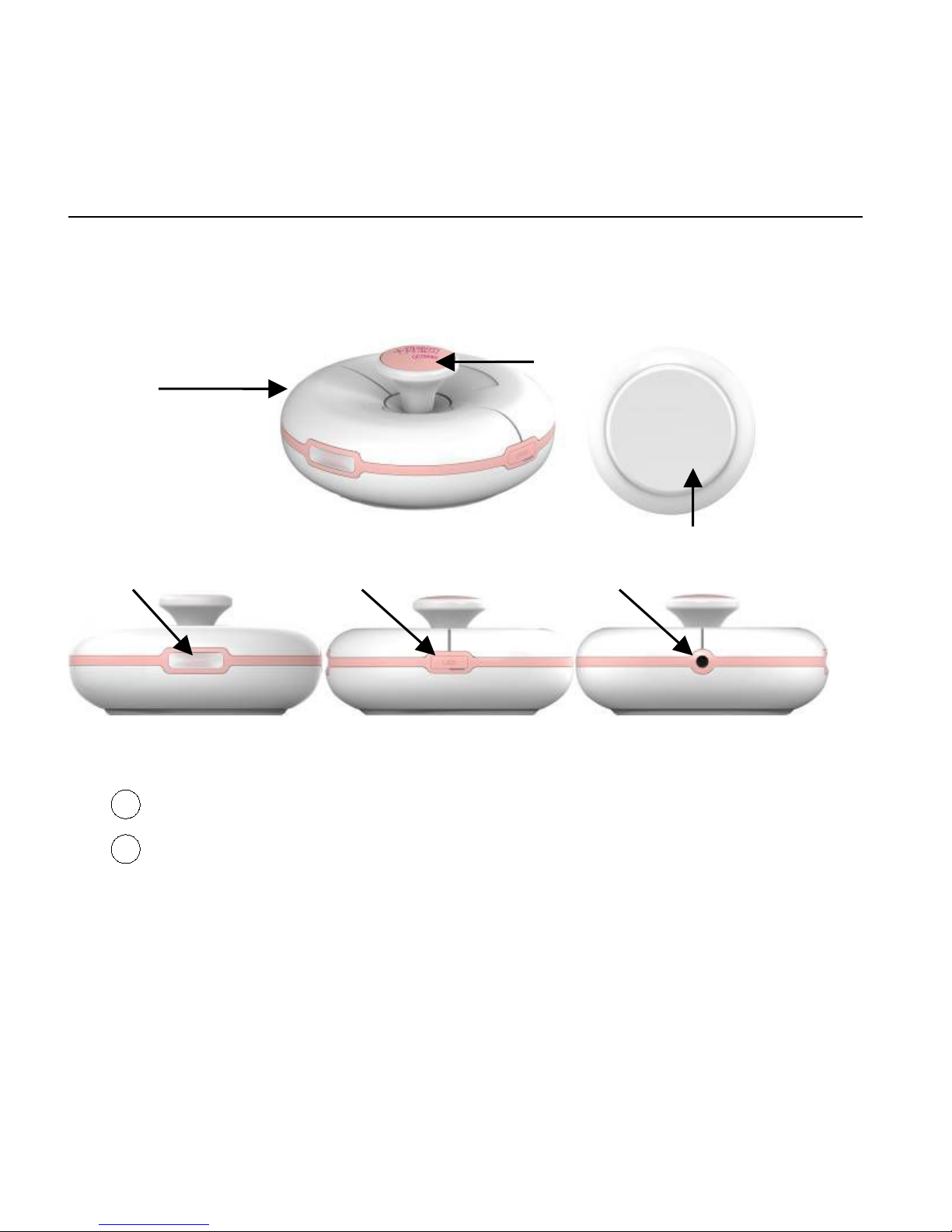
iFM-10B Series Fetal Doppler User Manual
7
3 Transducer and software
3.1 Transducer
Ultrasound side
Fig 3-1 Transducer Appearance
1Transducer: Receive fetal heart signal
2FHR and power indicator
FHR (110bpm-160bpm), green
FHR(less than 110bpm, over 160bpm), yellow
Power recharge: orange flashing
①
③
④
②
⑤

iFM-10B Series Fetal Doppler User Manual
8
Power recharge finished: Green
3On/off switch
Press it for 5 seconds for power on and off the device, the
Bluetooth function on/off during the device on/off.
4Mini USB port: connect USB cable
⑤Audio output
3.2 Software
3.2.1 Software Introdution
This software runs at front-page of the smart phone. It can be
interrupted if shift to other software. Do not shift to other software
during working.
Name: OCT Baby; Version: 1.0; Platform: Android 2.3 or higher
version.
Note: There is no time limit for software running.
This software does not rely on other software and will not affect
other software( if there is Android upgrade notice, new version No.
will replace old version No.), if delete something, operating system
iFM-10B Series Fetal Doppler User Manual
9
will give confirmation notice.
Initial Version No. is 1.0; Luckcome provide hardware
maintenance only; Any software issue, go to Android website for
upgrade; Any software issue does not damage SD card; before
upgrade, user need to backup information on SD card as the
mother information is stored there. Then connect USB cable to
computer for data copy or backup. One smart phone can only be
used on one mother only at the same time. Before using, user need
to input own information, the mother can monitor fetal heart rate
at any place at any time and can store sound and data. It is very
convenient for using and storage of precious memory.
This softare complies with China Food and Drug Administration
25000.51-2010 regulations.
3.2.2 Software download
Visit www.luckcome.com, download software package. Then
install the software as per installation guidance. Before installation,
ensure Android system version is 2.3 or higher, Smart phone display

iFM-10B Series Fetal Doppler User Manual
10
resolution is 1280*720 or higher.
SD card space is over 50M space .
3.2.3 Software Interface
Click , enter software. New user will see following interface:
Fig 3-2 1st Interface
Input ”Due Date”
Input ”Name”
Click “Next”, go to
next interface
Service Agreement

iFM-10B Series Fetal Doppler User Manual
11
1)Please check and review “Service Agreement” firstly,
understand related service terms, then input “Due Date” and
“Name”. After input, see following interface:
Fig 3-3 Input “Due Date” Fig 3-4 Input “Name”

iFM-10B Series Fetal Doppler User Manual
12
Note: It is a must to input “Due Date”, because all the
monitoring data are named by gestation week; gestation
week come from Due Date.
2)Click “Next”, if user do not input ”Due Date” and “Name”, it
will prompt following interface: “Input “Due Date and Name”
Fig3-5 Input “Due Date” indication Fig 3-6 Input “Name” indication

iFM-10B Series Fetal Doppler User Manual
13
3)After input “Due Date” and “Name”, click “next”, see
“Bluetooth connection” interface. Click to activate
communication:
Fig 3-7 Bluetooth Connection
All wireless devices have distance limitation to ensure stable data
communication. Blue tooth distance limitation is less than 10meters;
we recommend less than 5 meters between this device and smart

iFM-10B Series Fetal Doppler User Manual
14
phone. Longer than 5 meters, sound output may become
non-continuous and will be interfered by other Bluetooth devices.
That is normal situation, not device failure.
When log in OCT Baby software, Bluetooth function at smart
phonies not on, it prompt “Connection failed”; During testing, if
smart phone run out of power, or is at far distance or user turn off
Bluetooth function, after 20 seconds, it will prompt “Bluetooth
device disconnected”;This software can only connect with
Luckcome Fetal Doppler. User can login again after exit of APP.
This APP software and fetal Doppler connect by Bluetooth,
Bluetooth delay is less than 1.5 seconds; if hardware is not
connected, software can not go to monitoring status; if smart
phone platform system error, OCT BABY software will suggest exit
to re-activate the system.
4)If connected, it will go to monitoring interface automatically.

iFM-10B Series Fetal Doppler User Manual
15
If no response, system will give message “Network error”.
Click to store the data; click again to stop storage.
Fig 3-8 Monitoring Interface
5) Click “Record”, go to record interface to view the record.

iFM-10B Series Fetal Doppler User Manual
16
Fig 3-9 Record Review
Fig 3-10 Share

iFM-10B Series Fetal Doppler User Manual
17
Fig 3-11 Antenatal Training Music
6)Click , go to music interface ( see Fig 3-11). User
can listen to built in music; or copy favorable music to this folder
for listening.
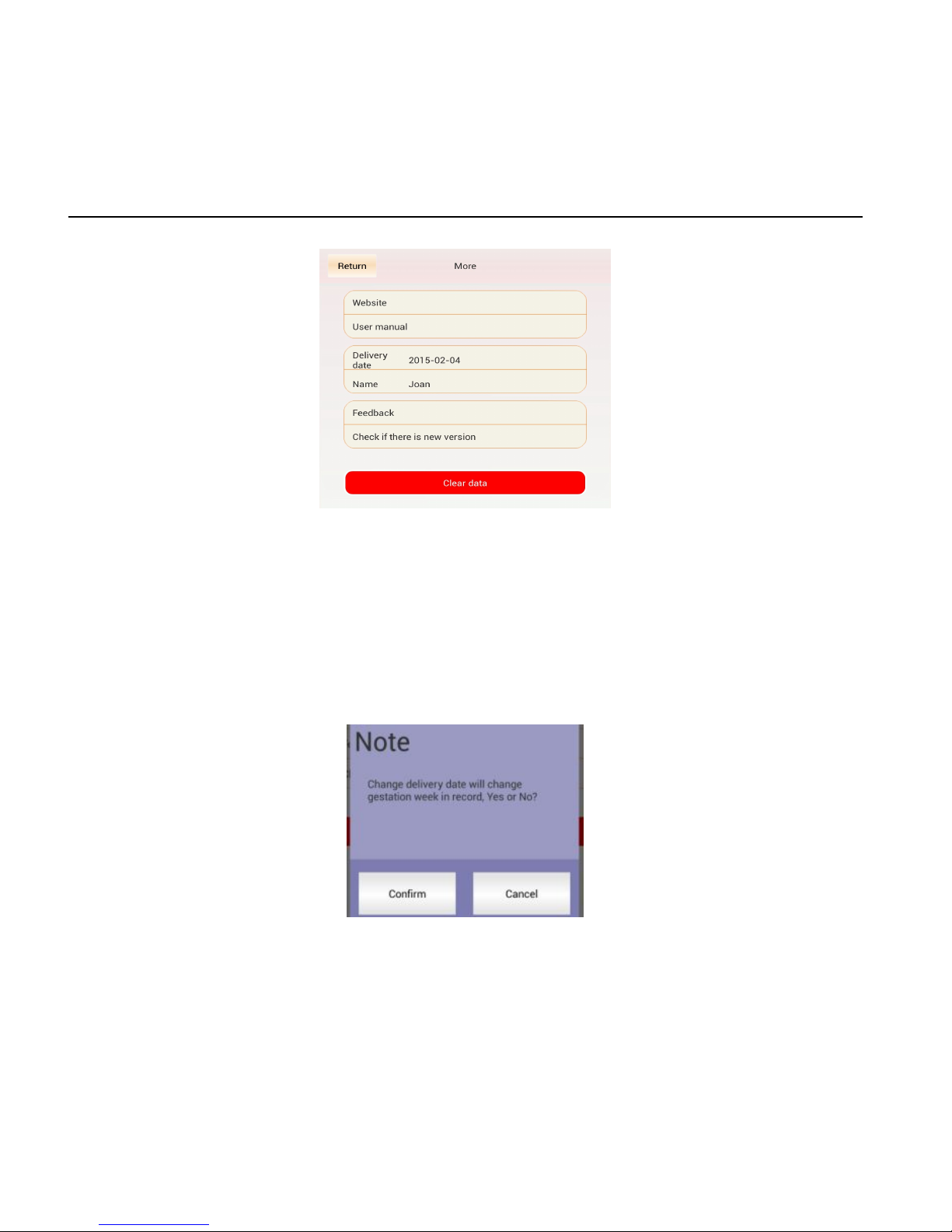
7)At monitoring interface, Click , go to “More”:
iFM-10B Series Fetal Doppler User Manual
18
Return----Go back to monitoring interface
Website----Go to www.luckcome.come
User Manual----Open User Manual( PDF format)
Delivery Date---- Display preinput delivery date, clickitif you
want to revise the delivery date.
After delivery, gestation week at history record will change.
Name: Display pre input name; click it if you want to revise the

iFM-10B Series Fetal Doppler User Manual
19
name.
Feedback --- Go to email interface to write email.
Check and update --- Check software version. If there is new
softwre, message prompt to remind user for update
software.
Click “Yes” to update software. After updating, smart
phone will auto reactivate the new software; if user does
not want to update, click “cancel”.
Clear the data ----- Delete hisotry data( Name, Delivery Date,
Record)
Table of contents