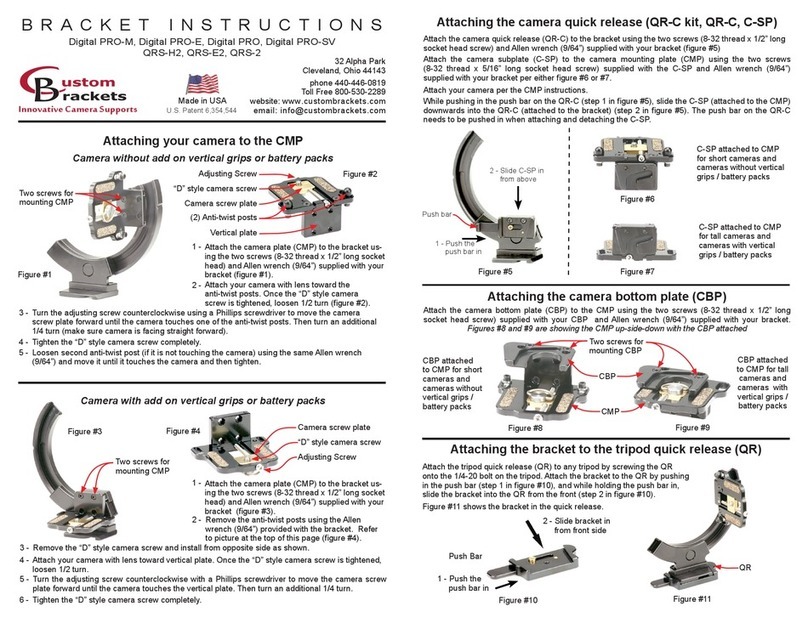
2.4 Principle of preventing liquid leakage
a. Complete sealed structure
VRLA battery is structurally completely airtight under the normal atmosphere. Especially,
vent hole is mechanically tight by valve and the safety valve was mounted with pressure
adjusting function if excessive pressure is filled inside of the battery taking into account of
safety.
b. Free move prevention structure of electrolyte
The electrolyte inside of battery is completely absorbed by glass fiber mat or gel which
absorption of liquid is very good and cannot freely move within the battery. Since therefore,
there is no worry about leakage of liquid as there is no electrolyte freely move even if the
battery is laid down or upside down.
2.5 Principle of self-electric discharge
The self-electric discharge phenomenon of lead-acid battery is by interaction between
active material of negative pole and electrolyte and the element incurs self-electric
discharge are largely classified into two(2) sorts as follows:
(a) Constituents consist electrolyte (hydrogen ion in sulfuric acid)
(b) Impure substances contained in electrolyte
Out of the two sorts above a is unremovable element but (b) is removable but (a) that is to
say the electrolyte itself cannot be eliminated and so, the electric discharge phenomenon by
lead battery cannot completely be restrained. However, electric discharge volume may be
reduced. VRLA battery has very small electric discharge volume than the existing lead
antimony battery and this is because of use of alloy of lead & calcium in lieu of alloy of lead
& antimony as the grid material. The plate alloyed by lead & calcium has very big resistance
against electric discharge. On the alloy grid surface of lead & calcium the sponge lead
which is negative pole active material is not easily discharged electricity since hydrogen is
difficult to be generated (high hydrogen over voltage)
For your information the electric discharge principle by hydrogen ion in electrolyte is as
follows:
(-) pole - hydrogen ion in electrolyte
2H+ + 2e ---→ H2(gas) ------------------------- (1)
(-) pole - lead which is active material
Pb + H2SO4 --→ PbSO4 + 2H+ + Ze -------------- (2)
To sum up (1) reaction & (2) reaction
Pb + H2SO4 --→ PbSO4 + H2 ↑
(charged condition) (discharge condition)
3. BEFORE USING
Please read following specification before using.
When we release the battery to the users, the battery keep initial charged state which can be
generated of flammable gas. Don't short each connection before sufficient ventilation of
Installed places and just keep it away from fire in order to prevent dangerous factors.