ATYS MEDICAL Microflow-S Operator's manual

Microflow-S
User’s Documentation

Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 2/12
This document contains all information on the Atys MICROFLO S devices manufactured b At s.
Information in this document is subject to change without notice and does not represent a commitment on the
part of At s.
At s does not assume liabilit for damages that ma occur because of the use of the information in this
manual.
No part of this manual ma be reproduced or transmitted in an form for an purpose other than the use of the
purchaser of Atys MICROFLO S.
1
S mbols ........................................................................................................................................................ 3
1.1
Device................................................................................................................................................... 3
1.2
Packaging ............................................................................................................................................. 3
2
Models & accessories................................................................................................................................... 4
3
Application specification ............................................................................................................................... 4
3.1
Intended medical indication .................................................................................................................. 4
3.2
Intended patient population .................................................................................................................. 4
3.3
Intended part of the bod or t pe of tissue applied to or interacted with.............................................. 4
3.4
Intended user profile............................................................................................................................. 4
3.5
Intended conditions of use.................................................................................................................... 4
3.6
Expected service life............................................................................................................................. 4
3.7
Contact duration on applied parts and accessible parts ...................................................................... 4
3.8
Contraindication.................................................................................................................................... 4
3.9
Essential performance.......................................................................................................................... 5
4
CAUTION...................................................................................................................................................... 5
4.1
Operator................................................................................................................................................ 5
4.2
Storage environment ............................................................................................................................ 5
4.3
Operating environment ......................................................................................................................... 5
4.4
Electrical safet .................................................................................................................................... 5
4.5
Maintenance and service...................................................................................................................... 6
4.6
Ultrasound field..................................................................................................................................... 6
4.7
Environmental protection...................................................................................................................... 6
4.8
Electromagnetic compatibilit ............................................................................................................... 6
5
Standard and regulation ............................................................................................................................... 7
5.1
Qualit management ............................................................................................................................ 7
5.2
Regulation............................................................................................................................................. 7
5.3
Safet and performance ....................................................................................................................... 7
5.4
Ultrasound ............................................................................................................................................ 7
5.5
Usabilit ................................................................................................................................................ 7
5.6
Risk management................................................................................................................................. 7
5.7
Electromagnetic compatibilit ............................................................................................................... 7
6
Environmental data....................................................................................................................................... 8
6.1
Batter .................................................................................................................................................. 8
6.2
Ph sical specifications.......................................................................................................................... 8
6.3
Doppler sound output ........................................................................................................................... 8
7
Operating ...................................................................................................................................................... 9
7.1
Theor of operation .............................................................................................................................. 9
7.2
Device description ................................................................................................................................ 9
7.3
CLEANING ........................................................................................................................................... 9
7.4
BATTERY REPLACEMENT ............................................................................................................... 10
8
Service........................................................................................................................................................ 10
8.1
Microflow S spare parts ...................................................................................................................... 10
8.2
Probe warrant ................................................................................................................................... 10
8.3
Assembl ............................................................................................................................................ 10
8.4
Test..................................................................................................................................................... 11
8.5
Probe connector wiring....................................................................................................................... 11
9
Document revision histor .......................................................................................................................... 11

Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 3/12
1 Symbols
1.1 Device
04959
DEEE Directive
2012/19/UE
General medical device
Directive 93/42/EEC
Separate collection for
Electrical Electronic Equipment
CE mark with number of the
Notified bod
ISO7000-434
IEC60417-5077
Consult accompan ing
documents.
HEADPHONES
IEC60417-5333
IEC60417-5134
IEC60417-5109
Applied part t pe BF
ESD sensible
Not for home use
ISO7000-2498
ISO7000-2493
EN980
Serial number
Catalogue reference number
Manufacturer
ISO 7000-2497
ISO7010-M002
Follow operating instructions
With manufacturing ear
IEC60417-5009 Stand b
Batter level. Blink when
low.
Sound volume adjust
IEC60417-5134
Electrostatic sensitive device
1.2 Packaging
ISO7000-626
ISO7000-2606
ISO7000-621
ISO7000-2620
ISO7000-632
Keep dr
Do not use if package is
damaged
Fragile
Limitation of relative humidit
Limitation of temperature

Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 4/12
2 Models & accessories
08 540 Module MICROFLO S
Detachable component and applied part:
13 496 C D 4MHz non preamplied probe L1.0m
Medisnap6
Detachable component and applied part:
13 503 C D 8MHz non preamplied probe L1.0m
Medisnap6
3 Application specification
3.1 Intended medical indication
The MICROFLOW S is intended for detection of blood flow in veins and arteries and as an aid for the
diagnosis of peripheral arterial disease and venous insufficienc .
3.2 Intended patient population
Adult onl .
3.3 Intended part of the body or type of tissue applied to or interacted with.
The detachable parts of the MICROFLOW S are applied on the patient’s skin mainl of the limbs, fingers, toes
and neck.
3.4 Intended user profile
The device must be used b or on the order of ph sician
3.5 Intended conditions of use
The device shall be used in the specified environmental operating conditions
3.6 Expected service life
½ hour per da
200 da s/ ear during 5 ears
½ ear for probes
3.7 Contact duration on applied parts and accessible parts
Probe’s active surface.
Contact time on the applied part on the same bod place: less than 1mn.
3.8 Contraindication
Never use the probe on skin surfaces with recent wounds/operative cuts.
Never allow the transducer to come in contact with bod fluid
It is not intended for foetal use
It not intended for use around e es

Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 5/12
3.9 Essential performance
Main performances are:
Doppler sound
Detection of flow/backflow for peripheral arteries using LED’s.
Loss or alteration of performance for these 2 items cannot result in injur .
Then there is no essential performance for this device documentation
4 CAUTION
4.1 Operator
The MICROFLO S must be used b or on the order of ph sician.
Cleaning and disinfection procedure must be applied between patients.
The MICROFLO S shall be used b trained people.
MICROFLO S is not intended to replace other means of evaluating vital patient ph siological
processes.
4.2 Storage environment
Climatic environment: storage: 10-40°C, 10-80% Hr.700-1060 Hpa
For transport and storage, the device must be placed in its original packing. Cautions to be applied
for the transport and the storage are labeled on the box.
If ou do not have the original packing materials, please contact our At s dealer.
Do not use the MICROFLO S if the packing of the device or of the probe is damaged.
The MICROFLO S must be stored and moved in its box.
The complementar protective packing must be used in the case of a dispatch.
4.3 Operating environment
Climatic environment: operating: 15-25°C, 10-80% Hr, 700-1060 Hpa
Do not use the device outside the specified environment.
To prevent fire and electrical hazards, keep the MICROFLO S out of rain, water and humidit . If
the s stem does come in contact with liquid, shut the s stem down and contact our At s service
representative.
The MICROFLO S shall not be used at home environment
The MICROFLO S must not be used outside the specified environment.
4.4 Electrical safety
Before use, control the sensor and its cable for visible damages. Never use the sensor if cracks or
other damages are visible.
Use onl the batter supplied b At s.
To change the batter , follow instructions delivered into user documentation.
In case of no use of the device for some time, remove the batter to prevent batter leakage.
Do not use after fulid insertion
Use onl the probes supplied b At s

Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 6/12
4.5 Maintenance and service
In case of breakdown of the MICROFLO S, please contact our At s dealer.
The MICROFLO S performs properl onl when operated and maintained as specified in this
manual.
It is the responsibilit of the operator to use the MICROFLOW S in accordance with the user’s
documentation, the warnings and the labels.
If the MICROFLO S is found defective, it should not be used. The MICROFLOW S should not be
used if an parts are missing or are damaged. Parts that are visibl broken, worn out, warped or
contaminated must be replaced.
No components should be replaced with parts from an other manufacturer. If the customer
suspects a part ma be defective, it is the customer’s responsibilit to contact At s or At s
representative. The MICROFLO S should onl be repaired b technicians authorized b At s.
No modification of this equipment is allowed
4.6 Ultrasound field
Contraindication: to be used b trained people, not use on ophthalmic, fetal application and fetal
monitoring.
The operator should limit the length of the Doppler tests to the time required for diagnostic purpose
to minimize his/her exposure to ultrasound and as well the patient’s.
4.7 Environmental protection
Do not dispose the MICROFLO S and its accessories in rubbish bins. The can be partiall
recovered and re-used.
4.8 Electromagnetic compatibility
Use of this equipment adjacent to or stacked with other equipment should be avoided because it
could result in improper operation. If such use is necessar , this equipment and the other
equipment should be observed to verif that the are operating normall .
Use of accessories, transducers and cables other than those specified or provided b the
manufacturer of this equipment could result in increased electromagnetic emissions or decreased
electromagnetic immunit of this equipment and result in improper operation.
The device shall not be used in home environment
NOTE The emissions characteristics of this equipment make it suitable for use in industrial areas
and hospitals (CISPR 11 class A). If it is used in a residential environment (for which CISPR 11
class B is normall required) this equipment might not offer adequate protection to radio-frequenc
communication services. The user might need to take mitigation measures, such as relocating or
re-orienting the equipment.
Portable RF communications equipment (including peripherals such as antenna cables and
external antennas) should be used no closer than 30 cm (12 inches) to an part of the Microflow-
S, including cables specified b the manufacturer. Otherwise, degradation of the performance of
this equipment could result.
The Microflow-S emits electromagnetic perturbations in ultrasound probe working frequencies,
fundamental and third harmonics.
The effects result from the high voltage and high frequenc emission pulses.
These perturbations do not allow to be compliant to the B class in ever configuration

Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 7/12
5 Standard and regulation
5.1 Quality management
The product is designed produced and serviced in compliance with ISO13485 requirements.
5.2 Regulation
EC: Class IIa
CE 0459
The package must be wasted according the national regulations.
The device must be wasted according the national regulations.
Do not waste the MICROFLOW-S and its accessories. The can be partiall recover and re-used.
5.3 Safety and performance
The device is compliant with IEC60601-1 2005/A1:2012 international standard of safet .
Safet class:
Class Internall powered
Applied parts: Doppler probes are BF t pe.
Mechanical protection index: IP20.
Water leak tightness height of the Doppler probes: 20 mm.
5.4 Ultrasound
Devices are compliant with IEC60601-2-37.
Ultrasound data on probes, TIB and TIS not require to be displa ed. (Below the limit)
5.5 Usability
The usabilit is processed in compliance with IEC60601-1-6
5.6 Risk management
The risk management is processed in compliance with ISO14971
5.7 Electromagnetic compatibility
The Microflow-S has been designed to work normall in conditions specified b the international standard
IEC60601-1-2: 2014
Information 1
Refer to the essential performance on this document.
Information relative to the A class limitation
The MICROFLO S emits electro-magnetic perturbations in ultrasound probe working frequencies,
fundamental and third harmonics.
The effects result from the high voltage and high frequenc emission pulses.
These perturbations do not allow to be compliant to the B class in ever configuration
Table 1
Manufacturer’s Declaration – Electromagnetic Emissions (IEC60601-1-2)
The Microflow-S is suitable for use in the specifie electromagnetic environment. The customer an /or user shoul
assure that it is use in an electromagnetic environment as escribe below;
Emission Test Compliance Compliance Electromagnetic Environment
RF emission
CISPR 11
Group 1 The Microflow-S must emit electromagnetic energy in or er to perform its
inten e function.
Nearby electronic equipment may be affecte .

Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 8/12
Emissions RF
CISPR 11
Class A The emissions characteristics of Microflow-S make it suitable for use
in industrial areas and hospitals (CISPR 11 class A).
If it is used in a residential Microflow-S might not offer adequate
protection to radio-frequenc communication services.
The user might need to take mitigation measures, such as relocating or
re-orienting this equipment.
Table 2
Manufacturer’s Declaration – Electromagnetic Immunit (IEC60601-1-2)
The Microflow-S is suitable for use in the specified electromagnetic environment. The customer and/or user
should assure that it is used in an electromagnetic environment as described below;
Immunity Test Test Level
IEC 60601-1-2
Compliance
Level
Electromagnetic
Environment Guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 8 kV contact
± 2 kV ± 4 kV ± 8
kV ± 15kV air
± 8 kV contact
± 2 kV ± 4 kV ± 8
kV ± 15kV air
Floor should be antistatic, wool. If floor are
covered with s nthetic materials, the
relative humidit should be minimum at
least 35%.
Table 3
Manufacturer’s Declaration - Electromagnetic immunit
The Microflow-S is suitable for use in the specified electromagnetic environment. The customer and/or user
should assure that it is used in an electromagnetic environment as described below;
Immunity
Test
IEC 60601 test
level
Compliance
level
Electromagnetic environment - guidance
Radiated RF
IEC 61000-4-3
3 V/m
80 MHz to 800
MHz
3 V/m
800 MHz to 2.5
GHz
3 V/m
3 V/m
Portable and mobile RF communications equipment
should be used no closer to an part of the
Microflow-S including cables, than the
recommended separation distance of 30cm or 12
inches.
Note 1: 80 MHz to 2.7GHz, the higher frequenc range applies.
Note2 : These guidelines ma not appl in all situations. Electromagnetic propagation is affected b
absorption and reflection from structures, objects, and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radio, AM and FM radio broadcast, and TV broadcast cannot be
predicted theoreticall with accurac . To assess the electromagnetic environment due to fixed
RF transmitters, an electromagnetic site surve should be considered. If the measured field
strength in the location in which the Microflow-S is used exceeds the applicable RF compliance level
above, the Microflow-S should be observed to verif normal operation. If abnormal performance is
observed, additional measures ma be necessar , such as re-orienting or relocating the Microflow-S.
Over the frequenc range 150 kHz to 80MHz, field strengths should be less than 3 V/m
6 Environmental data
6.1 Battery
Battery type: 9 V alkaline - 6LR61 or PP3 not rechargeable
Battery life: 5h charge.
6.2 Physical specifications
Casing Width = 80 mm
Depth = 150 mm
Height = 40 mm
Weight 200 g to 300g with batter and probe
6.3 Doppler sound output
Outpour power: 500mW RMS
Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 9/12
7 Operating
7.1 Theory of operation
The 4 MHz and 8 MHz transducers are used to examine the arteries and veins of the upper and lower limbs as
well as to examine the vessels suppl ing the brain, enabling vascular disease to be evaluated quickl and
easil .
The Doppler principle is used routinel to transcutaneousl detect the motion of red blood cells. The probe is
placed against the skin nearest to the target vessel.
One cr stal emits 4 or 8 MHz frequenc . When sound waves strike the moving blood cells, parts of them are
reflected towards the transducer. The reflected signal has a different frequenc than the emitted signal. This
frequenc difference is known as the Doppler shift. It is proportional to the blood velocit .
The equipment amplifies the frequenc change and channels it to the speaker.
It also calculates the frequenc shift and displa s it on 6 LEDs, 3 red l LEDs for the flow and 3 blue LEDs for
the backflow.
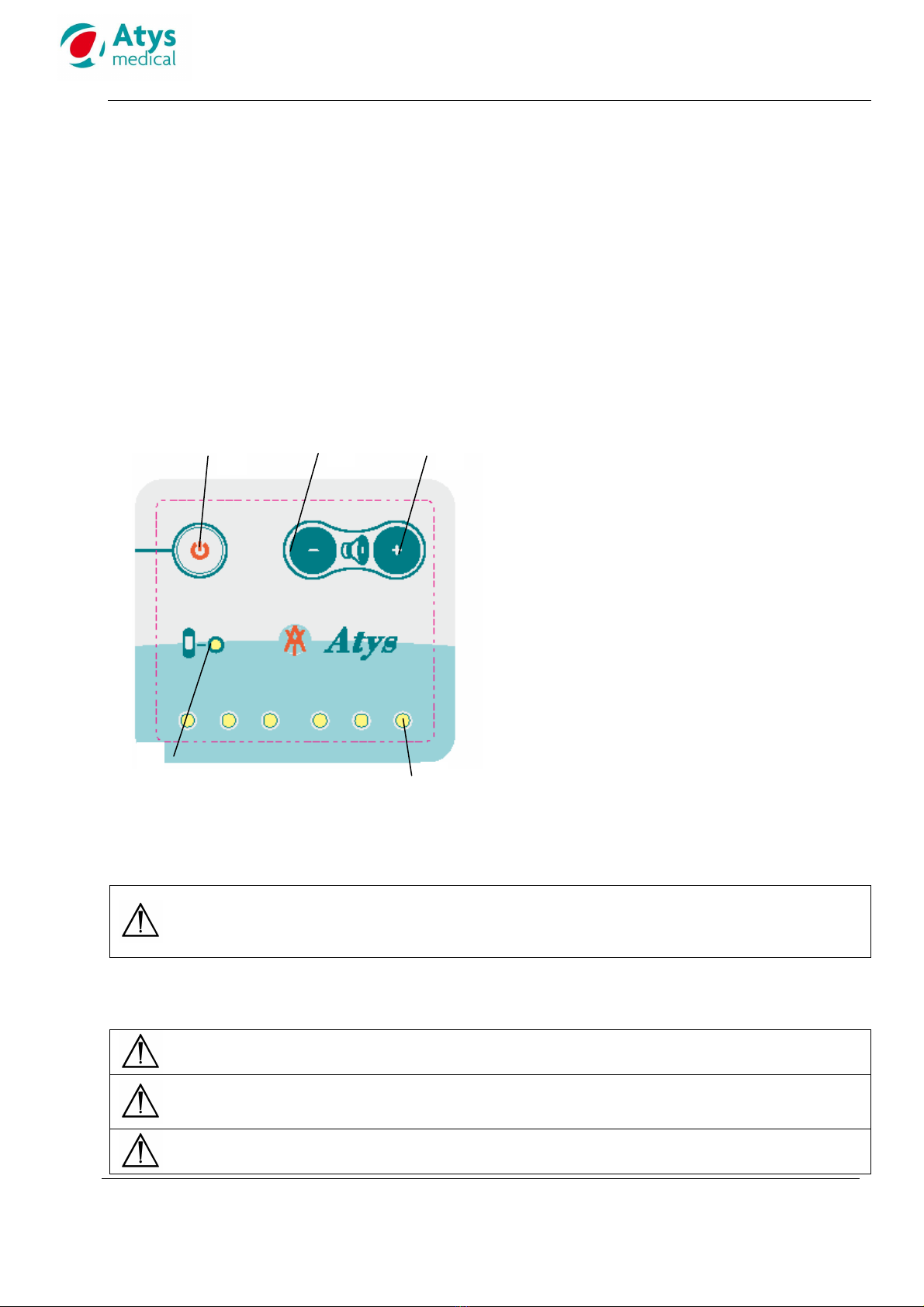
7.2 Device description
1: Power on/off button. Auto power off after 5mn
without signal
2: Volume down button
3: Volume up button
4: Yellow LED.
- Lights continuousl when the batter is
operational
- Flashes if the batter is low.
5: Three red LEDs indicate flow towards the
transducer and three blue LEDs flow awa from
it.
The number of lightning LEDs is related to the
flow intensit .
Since the device is bi-directional, the red and blue
LEDs might light simultaneousl . This would
indicate that an arter and a vein have been
located at the same time.
The seven LEDs light up during 1 second when the
device is switched on.
The probe is used with a gel for ultrasound.
7.3 CLEANING
Casing cleaning: The panels of the device can be cleaned with a soft cloth dampened with alcohol.
Alwa s turn off the s stem before cleaning the machine. Otherwise, electric chock ma result.
Do not place fluid on or near the s stem.
Make sure that the cloth is damp but not saturated, as ou should avoid introducing fluids into
areas of electrical components
Probe cleaning: The probes must be cleaned after each use with a usual mild disinfectant solution.
As the probe comes in contact onl with intact skin, the risk of infection is low; so the probe and the probe
casing need onl to be cleaned and low-level disinfected between patients.
Never place the probe over open wounds or allow it to encounter bod fluid.
Be ver careful for the cleaning of the probe.
It must be handled carefull .
Never bend or pull the cable.
Use onl hospital approved cleaning agents (for example 70-90% isoprop l alcohol) to clean the
probe and probe casing after each use and wipe dr immediatel .
1
2
4
5
3
Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 10/12
Do not immerse the probe casing.
Do not clean the probe with acetone, eth l alcohol or sodium h pochlorite (bleach) as this will
damage the surface
Avoid an cleaner that ma scratch or dissolve plastic surface
High level disinfection using liquid agent.
The probes can be sterilised with peracetic acid solution (for example Aniox de).
Soaking duration: 20 min
Leak tight length of the probes: 20 mm
After cleaning
The proper state of the probe must be checked after sterilisation: a visual checking of the extremit of the
probe must be performed. There must not be an cracks or upheaval.
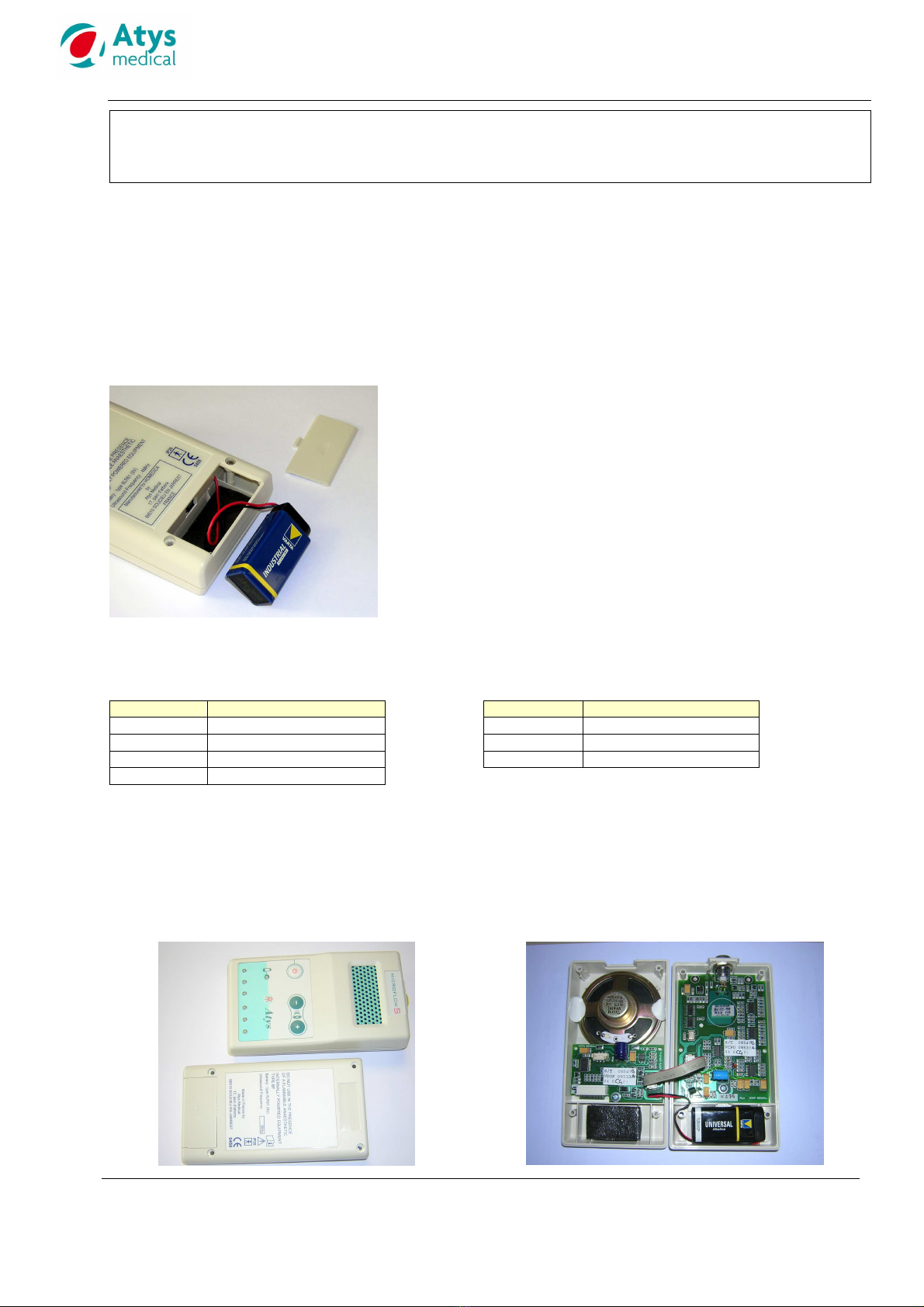
7.4 BATTERY REPLACEMENT
The batter should be replaced as soon as the ellow
LED flashes
Respect the batter t pe: 9 V alkaline - 6LR61 or PP3
Be careful about the polarit when replacing the
batter .
8 Service
8.1 Microflow S spare parts
Order number
designation Order number
designation
04 739 Loud speaker 11 830 Microflow S: ke board
04 753 Microflow grid 11 837 Labels
08 470 Casing 04 746 Plastic box
08 512 Electronic board
8.2 Probe warranty
The pencil probes are warranted for six (6) months against manufacturing defect.
Limitation of the duration of the probe warranty in the case of liquid sterilisation
The warrant of the pencil probes is limited to 50 sterilisation periods of 20 min each.
8.3 Assembly

Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 11/12
8.4 Test
Connect a new batter .
Switch on the unit: all the leds must switch on and then off.
Activate the probe: a clear sound should be eared.
Ke board: does the sound volume control work?
Switch off the unit.
8.5 Probe connector wiring
9 Document revision history
Reference: 20 174 C MICROFLO S User’s documentation
March, 2017
Atys
17 Parc d’Arbora
F69510 SOUCIEU EN JARREST
France
Tel: 33 4 78 05 69 69
Fax: 33 4 78 05 69 60
Change # R. date Description
04 272 A March 16, 2012 Initial release
05 371 B Januar , 2017 Phase 401 revision
05 518 C March, 2017 Accessories reference update
Issued b
Benoît Guibert
Approval Christine Turlat

Microflow-S pocket Doppler
User’s Documentation
20 174 C
Page 12/12
DECLARATION OF CONFORMITY / DECLARATION DE CON ORMITE
Directive 93/42/EEC / Directive 93/42/CEE
Manufacturer’s Name: Atys
Nom du fabricant :
Manufacturer address: 17 Parc d’Arbora
Adresse du fabricant : 69510 SOUCIEU EN JARREST, FRANCE
Product name: Microflow-S
Nom du produit :
Model name: Microflow-S
Nom du modèle :
Product category: Electromedical devices
Doppler non-imaging ultrasound systems for blood flow
measurement, associated probes
Catégorie du produit : Dispositifs électro médicaux
Systèmes Doppler à ultrasons sans imagerie pour la mesure
des flux sanguins, sondes associées associés
Classification (per Annex IX): Class IIa
Classification (Annexe IX) :
Conformity Route: Annex II section 3
Annexe : Annexe II point 3
e herewith declare that the above mentioned product meets the provisions of the Council
Directive 93/42/EEC for the Medical devices. All supporting documentation is retained under
the premises of Atys, 69510 Soucieu en Jarrest, FRANCE.
Nous certifions que le produit mentionné ci-dessus est conforme aux exigences de l’annexe II point
3 de la Directive 93/42/CEE pour les dispositifs médicaux. Atys tient un dossier technique à la
disposition des Autorités compétentes.
Notified Body: LNE
Organisme notifié : 1, rue Gaston Boissier
75724 PARIS cedex 15, FRANCE
ID#0459
EC certificate: 7761
Certificat CE :
Date:
Signature: ____________________
Benoît Guibert
Quality Manager / Responsable qualité
Table of contents
Popular Personal Care Product manuals by other brands

Meditech Electronic
Meditech Electronic Audio-Trainer 3000 MAIN MANUAL

Remington
Remington Reveal Diamond Microdermabrasion MD3000 user manual

Posture Pump
Posture Pump Disc Hydrator 2000 instructions

HOT TOOLS
HOT TOOLS HT1095BG Use and Care Instruction Manual

HoMedics
HoMedics ParaSpa SELECT instruction manual

Beurer
Beurer cellulite releaZer Instructions for use