Bausch OccluSense User manual

INITIAL SETUP
In the OccluSense®-iPad-App select ‘Settings’ and tap
on ‘Initial setup’.
Switch on the handheld.
(press the pink operating button 1)
Your handheld first creates its own network (SSID)
which is shown with the password (WEP) in the
display.
Tap 'Start' in the OccluSense®-iPad-App to initiate
the setup process and type the WEP password into
the dialog box.
Confirm the following dialog with ‘Join’.
WLAN SETUP
Confirm the following dialog with ‘Yes’ to connect the
OccluSense®handheld device with your WLAN.
A screen opens with three steps to be performed.
Tap on ‘Next’ to start the process. Now enter your
network name (the last selected SSID is suggested)
and your WLAN password.
Once the data has been entered, tap ‘Next’.
The OccluSense®-iPad-App establishes a connection
to the handheld via this network.
A successful connection between the handheld and
the wireless network is indicated by green signal bars
and the first 6 characters of the wireless network in
the upper left corner of the handheld display.
If the connection between the handheld and the
wireless network has failed, the connection status is
indicated on the handheld display by transparent
signal bars. In this case reset the handheld (manual
chapter 19.2) and start the initial setup again.
Quick Start Guide
PREPARATION
Make sure your iPad is connected to your wireless
network and that your wireless network supports the
2.4 GHz (IEEE802.11 b/g/n) frequency. Have your
WLAN password ready.
Install the OccluSense®-iPad-App
www.occlusense.com/install
1
23
PRI_IFU_OccluSense_QS_EN_002_200110.qxp_Layout 1 Kopie 24.01.20 14:55 Seite 1

REGISTRATION OF THE SYSTEM
Before registering your system, make sure you have
an internet connection.
We recommend to register your system immediately
to ensure full functionality and extended use. If this
will not be done, the system will automatically return
to demo mode after 14 days.
To register your system, open the OccluSense®-iPad-
App.
Tap on ‘Settings’ in the menu.
Then go to ‘Handheld registration’.
Fill in the registration form. Please fill in the following
fields:
Country, name, first name, street, postal code, city
and your email address.
Tap the ‘Register’ button to send your registration
data to your e-mail address.
A confirmation email will be sent to your email
address, which you must open and confirm.
Restart the OccluSense®-iPad-App. If your registra-
tion was successful, a green checkmark will appear
in the OccluSense®-iPad-App.
Your system is now registered and ready to use.
TECHNICAL REQUIREMENTS FOR THE
WIRELESS LOCAL NETWORK (WLAN)
• 2.4 GHz WLAN-Standard IEEE802.11 b/g/n
(5 GHz is NOT supported)
• No MAC filter activated
(MAC = Medium Access Control)
• DHCP server with two free IP addresses available
OccluSense®is a network device - If you have any
problems connecting the OccluSense®to your
network, please contact your system administrator.
SUPPORT
help.occlusense.com
Multilingual Phone Support
Europe
+49-221-98259010
The Americas
+1 (844) 633-4002
Japan
+81-50-3101-4161
PRI_IFU_OccluSense_QS_EN_002_200110
4 !
?
PRI_IFU_OccluSense_QS_EN_002_200110.qxp_Layout 1 Kopie 24.01.20 14:55 Seite 2

Instructions for Use

Bausch and OccluSense®are trademarks of Dr. Jean Bausch GmbH & Co. KG, registered in the EU and other countries.
Apple, Mac, iPad, iPad Air, iPad mini, AirDrop, iTunes are trademarks of Apple Inc.,
registered in the U.S. and other countries.
All other trademarks are the property of their respective owners.
2

3

INDEX
Symbols..............................................................................................................................8
Terminology, Hardware requirements iPad................................................................9-10
GENERAL INFORMATION
1 Medical products manufacture....................................................................................11
2 Medical products distributors......................................................................................11
3 Intended Use...........................................................................................................12
4 Device description (components, etc.)....................................................................12
4.1 Operating and functional elements of the OccluSense-System........................13
4.2 System features...............................................................................................14
5 Contraindications.........................................................................................................15
6 Expected clinical benefit.........................................................................................15
7 Precautions / Safety Instructions............................................................................16
PREPARATION
8 Installation of the OccluSense®-iPad-App ..................................................................17
8.1 Battery Charging......................................................................................18-21
OccluSense®-iPAD-APP
9 Overview..................................................................................................................22
9.1 Intended use of the OccluSense-iPad-App......................................................22
9.2 Overview of the OccluSense-iPad-App.............................................................22
9.3 User manual.....................................................................................................23
INSTALLATION
10 First Use...............................................................................................................24
10.1 Demo Mode...................................................................................................24
10.2 Initial Setup.................................................................................................25
10.2.1 Switch on the handheld................................................................................25
10.2.2 Start the initial setup..............................................................................26-27
10.2.3 Integration into your own WLAN network.....................................................28
10.2.4 Registration of the system.......................................................................29-30
10.3 Administration of OccluSense handheld devices...........................................31
10.3.1 Rename handheld........................................................................................31
10.3.2 Disconnect handheld....................................................................................31
10.3.3 Pairing of the handheld with the OccluSense-iPad-App................................32
4

5
SAFETY
11 Safety Check...........................................................................................................34
12 Function test......................................................................................................34-35
PATIENT MANAGEMENT
13 Manage patient data...............................................................................................36
13.1 Overview..........................................................................................................36
13.2 Create patient..............................................................................................37
13.3 Edit patient data..........................................................................................38
13.4 Delete a patient’s data.................................................................................38
RECORDINGS
14 Start a recording....................................................................................................39
14.1 General........................................................................................................39
14.1.1 Switch off handheld.....................................................................................40
14.1.2 Insert Sensor...........................................................................................40-41
14.1.3 Switch on the handheld....................................................................................42
14.1.4 Check WLAN connection...............................................................................42
14.1.5 Check if sensor is ready for recording.............................................................43
14.2 Set a patient active.........................................................................................43
14.2.1 Patient data..................................................................................................44
14.3 Recording modes.............................................................................................45
14.3.1 Recording and Live-Mode........................................................................46-47
14.4 Recording of the masticatory pressure distribution......................................48
14.4.1 Insert the sensor into the patient’s mouth.......................................................48
14.4.2 Recording.....................................................................................................49
14.4.3 Finish recording.......................................................................................50-51
EVALUATION OF RECORDINGS
15 Viewing recordings.......................................................................................................52
15.1 Overview..................................................................................................52-55
15.2 Resizing, turning and tilting the view...........................................................55
15.3 Playing recordings........................................................................................56
15.4 Filtering.......................................................................................................57
15.5 Viewing recorded data...................................................................................58
15.6 Additional visualization options........................................................................59
15.7 Renaming recordings and adding notes........................................................60
15.8 Fullscreen........................................................................................................61

MANAGE RECORDINGS
16 Manage and export recordings................................................................................62
16.1 Play back recordings...................................................................................63
16.2 Compare recordings.................................................................................63-64
16.3 Export recordings and snapshots.................................................................64
16.4 Export recordings as video..............................................................................65
16.5 Export data via iTunes PC/Mac software.......................................................66
16.6 Export patient’s data via AirDrop..................................................................67
16.7 Import.............................................................................................................67
SETTINGS
17 Menu ”Settings”......................................................................................................68
17.1 Recording settings.......................................................................................68
17.2 Handheld firmware (and updates)................................................................68
17.3 Terms and conditions...................................................................................68
18 Menu “Imprint”.......................................................................................................68
TROUBLESHOOTING
19 Basic troubleshooting..................................................................................................69
19.1 Damages of the handheld............................................................................69
19.2 Reset to factory default....................................................................................69
19.3 Connection lost after Firmware Update........................................................69
19.4 Entering wrong WLAN credentials..................................................................69
19.5 Troubleshooting table..............................................................................70-73
CLEANING
20 Cleaning and disinfection..........................................................................................74
20.1 Handheld.........................................................................................................74
20.2 Charging Station..............................................................................................75
20.3 Test-Sensor....................................................................................................75
20.4 Reusability...................................................................................................75
MAINTENANCE
21 Batteries, electrical safety, software updates..........................................................76
21.1 General..........................................................................................................76
21.2 Replacing the batteries............................................................................76-77
21.3 Software-Updates..........................................................................................78
6

NOTICE
22 Transport & Storage................................................................................................78
23 Expected lifetime....................................................................................................78
24 Disposal Instructions................................................................................................78
25 Electromagnetic Compatibility.................................................................................79
26 Warrenty.................................................................................................................80
27 Customer Service....................................................................................................80
28 Spare parts...............................................................................................................81
29 Notification of incidents..........................................................................................81
30 Declaration of Conformity............................................................................................82
7
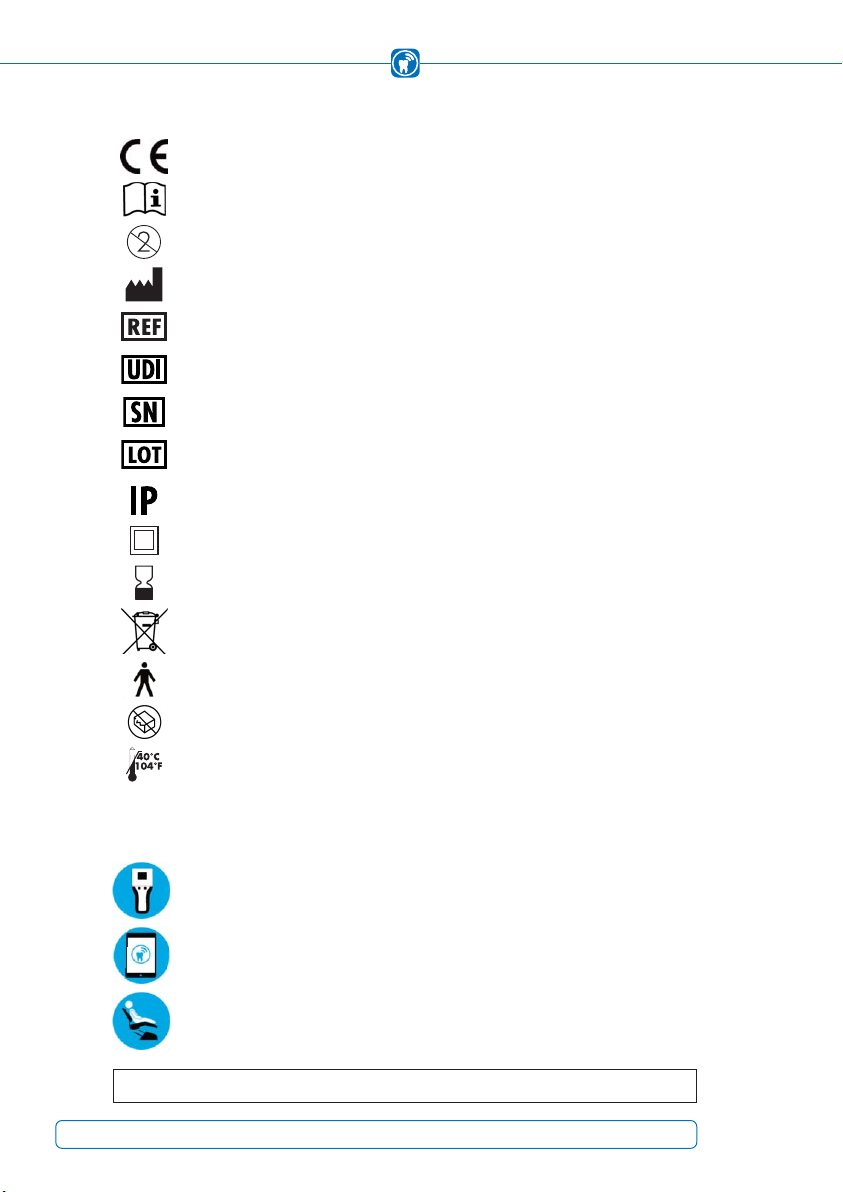
Symbols
Some chapters describe usage procedures. In order to see which action of the
OccluSense®-System is required, the following symbols are used:
In compliance with relevant EU directives
Follow Instructions for Use!
Do not reuse!
Medical Device Manufacturer
Order Number
Unique Device Identifier
Serial Number
LOT Number
IP Protection Class
Expiry date [yyyy-mm-dd]
Waste electrical and electronic equipment (WEEE) must be disposed of
separately. These must not be disposed of with normal household waste.
Applied part of TYPE B, IEC 60601-1
Do not use if package is broken!
Do not expose to heat!
User interaction with the handheld
User interaction with the iPad
Interaction with the patient
Class II
8
WEEE-Reg-No.: DE 11349707

Terminology
Recorded raw data:
Recorded data by the handheld device.
OccluSense®-iPad-App:
The software that manages, stores and visualizes the raw data recorded by the
OccluSense®handheld (Available for free in the Apple AppStore).
Recording:
Raw data converted by the OccluSense®-iPad-App.
OccluSense®-System:
Entire treatment unit according to chapter 4 "Device description".
Handheld:
The central electronic device of the OccluSense®-System, which executes the
collection of raw data as well as its intermediate storage and transmission to the
iPad (chapter 4.1).
Hardware requirements iPad
(not included)
Regarding the status of these operating instructions, an evaluation unit is an Apple iPad
on which the app “OccluSense®” from the Apple App Store must be installed. In order
to use the app, you first have to register the system within the app.
The OccluSense®-iPad-App has been developed for the following iPad models:
Installation on an iPad mini is not recommended. In order to use the app in full
functionality, the system must be registered first within the app. The instructions for
the installation and registration can be found in chapter 10.
iPad Air 2013
iPad Air 2 2014
iPad Pro 12.9" 2015
iPad Pro 9.7" 2016
iPad (5th gen.) 2017
iPad Pro (2nd gen., 12.9") 2017
iPad Pro (2nd gen., 10.5") 2017
iPad (6th gen.) 2018
iPad Pro (3rd gen., 11") 2018
iPad Pro (3rd gen., 12.9") 2018
iPad Air (3rd gen.) 2019
iPad (7th gen.) 2019
9
CAUTION
Using the application on a not supported device is possible but at the user’s•
own risk.
It is possible to run the app on an unsupported iOS version at the user’s own•
risk.
NOTE
A particular notification will pop up when the app is running on a not supported•
iOS or hardware.
The usage out of the application’s technical scope will be logged for safety rea-•
sons.
10

GENERAL INFORMATION
1 Medical products manufacturer
Dr. Jean Bausch GmbH & Co. KG
Oskar-Schindler-Str. 4
D-50769 Köln - Germany
Phone: +49-221-70936-0
Fax: ++49-221-70936-66
Web: www.occlusense.com
2 Medical products distributors
(U.S. Initial importer)
Bausch Articulating Papers, Inc.
12 Murphy Drive, Unit 4
Nashua, NH 03062 - United States of America
Tel: (603) 883-2155 | Tel: 1-888-6-BAUSCH | Fax: (603) 883-0606
Bausch Articulating Papers (Australasia) Pty. Ltd
G.P.O. Box 3733, Sydney NSW 2001, Australia
Tel: +61-2-9345-1945 | Fax: +61-2-9345-1955
Bausch Articulating Papers Japan K. K.
2nd Floor, 1-4-2 Jonan, Ikedashi, Osaka Japan 563-0025
Tel:+81 72-737-9501 | Fax:+81 72-737-9502
Bausch Importaão de Materiais Odontológicos Ltda.
Rua Paulo Eduardo Xavier de Toledo, 379 salas 8 e 9
13304-240 Itu-SP, Brasil
Tel: +55 11 3020-9263
11

3 Intended Use
Recording and presentation of the masticatory pressure distribution in order to localize
relevant pressure points of the patient's entire dental arch with full-grown dentures (for
temporary use only). The application is intended exclusively for dentists and
physicians/physiotherapists with specialized training (CMD, craniomandibular
dysfunction).
4 Device description (components, etc.)
The OccluSense®-System consists of the following components:
- Handheld BK 5001
- Charging station incl. Power supply BK 5002
- iPad App available in the Apple AppStore BK 5100
(requires an Apple iPad [no medical device], not included)
- 1 test sensor BK 5011
- 25 OccluSense®-Sensors BK 5025
- Manual BK 5051
- 3 NiMH rechargable batteries BK 5013
- 1 Screwdriver IP 6 BK 5012
The medical device components do not contain any of the following substances:
- Pharmaceuticals
- Tissues or cells of human origin or their derivatives
- Tissues or cells of animal origin or their derivatives within the meaning of
EU Regulation 722/2012
The touchable surfaces of medical device components consist of physiologically harmless
plastics and colorants.
Environmental conditions:
Temperature range 0° … +35°
Relative humidity 20% … 90%. noncondensing
Atmospheric pressure 650hPa … 1060hPa
max. height a.s.l. 3000m
International Protection Rating: IP 40
Class: Class II
12

4.1 Operating and functional elements of the OccluSense®-
System
1 – Charging station incl.
power supply 2 (accessory)
3 – Sensor (Applied Part type B)
4 – Handheld (ME-Device)
5 – Control button 1 (pink)
6 – Control button 2 (green)
7 – Sensor lid
8 – Display
9 – Unlock button
10 – Control button 3 (Reset)
11 – Battery compartment
12 – Test sensor (accessory)
(not shown)
13 – Screwdriver (accessory)
(not shown)
14 – 3 pieces AAA NiMH rechargeble
batteries (accessory)
(not shown)
13
1
2
345 6 78910 11

4.2 System features
The OccluSense®-System serves as an aid for recording and depicting of occlusal
conditions by presenting the pressure conditions of the contact points of the masticatory
force distribution. It does not generate therapy recommendations automatically.
Furthermore, the system does not control the treatment/therapy and there is no inter-
vention in vital functions.
The occlusal conditions are recorded by the handheld device with inserted sensor. The
recorded raw data, generated during the occlusion test, are stored locally on the hand-
held device. Subsequently the handheld transmits this data via wireless interface to the
OccluSense®-iPad-App. The OccluSense®-iPad-App saves and displays the received data
as a recording.
14
Electrical
connection
inductive
power transmission
(galvanically isolated)
Electrical
connection
WLAN
iPad
Power supply
Charger
OccluSense®
Sensor
OccluSense®
Handheld

5 Contraindications
The OccluSense®-System is for professional use only (see chapter 3) and is not suitable
for:
Patients with edentulous jaw•
Patients with incomplete full-grown jaw•
6 Expected clinical benet
Using this system it is possible to check the patient´s masticatory pressure distribution.
In comparison to traditional occlusion test materials (papers, foils), the system does
not only show the local premature contacts on the occlusal surfaces of the teeth, but
due to the OccluSense®-iPad-App it is possible to see a graphical representation of
occlusal pressure conditions on the iPad.
It is possible to visualize the proportion of the masticatory forces of the entire dental
arch and thus a support for the practitioner for further patient-oriented therapy planning
is provided. Several recordings can be compared, e.g. before and after dental treatment,
and can be saved on the iPad for documentation purposes.
Therapeutic measures must not be applied on the basis of the data of the OccluSense®-
System only, further information is required, in order to achieve a comprehensive diag-
nosis. The representation of the masticatory forces does not allow any conclusions
regarding the actual exerted forces (absolute values). These are relative value repre-
sentations.
15

7 Precautions/ Safety Instructions
OccluSense®is a medical device meant for professional use only (see chapter 3).
The OccluSense®-System must be used with the supplied or prescribed accessories only.
It is not allowed to connect any part of the OccluSense®-System to an external DC power
source. The power supply of the system has to be carried out exclusively with the
supplied charging accessory.
Before using the OccluSense®-System, components have to be checked for external
damages (visual inspection). Damaged components must not be used.
The OccluSense®-System must not be altered without the manufacturer's permission.
The sensor must not be modified (e.g. cut to size).
The handheld is designed for battery operation only. Mains operation is not intended
and may result in serious injury of the patient or the practitioner.
The use of non-rechargeable batteries is not permitted and may result in serious injury
of the practitioner or destruction of the handheld when attempting to recharge it.
The handheld must be disconnected from the charging station during use.
The charging station has to be located outside the patient´s vicinity.
The charging station must be positioned in such a way that the plug-in power supply
unit is in easy reach and the separation from the mains can be accomplished without
difficulty.
The charging station can be disconnected from the mains by pulling the plug-in power
supply out of the socket.
All parts of the OccluSense®-System are not thermo-disinfectable or autoclavable and
are not delivered sterile.
Only cleaning agents and disinfectants as described in chapter 21 „Cleaning and
disinfection“ are allowed.
The charger is maintenance-free.
The treatment light should not be directed towards the handheld during treatment to
ensure good readability of the display.
The OccluSense®-System is not intended to serve as the sole basis for diagnosis / the-
rapy decisions.
16

PREPARATION
8 Installation of the OccluSense®-iPad-App
Before using the OccluSense®-System for the first time, install the OccluSense®-iPad-
App on the iPad (www.occlusense.com/install).
Before initial operation of the OccluSense®-System or after reset to factory default, a
network configuration of the handheld and the associated pairing with an iPad is required.
The procedure is described below.
NOTE
The handheld comes with uncharged batteries. The rechargeble batteries must•
be charged before first use (see chapter 8.1).
The OccluSense®handheld is designed for use with three AAA NiMH batteries•
only (included). The handheld can also be used with other AAA NiMH batteries
than those supplied. These AAA NiMH batteries must have a minimum capacity
of 1000mAh.
Perform a visual inspection for external damages.•
Only use the handheld unit and charging station if there are no external dama-•
ges.
17

8.1 Battery Charging
2. Insert the battery compartment cover
and tighten the screws.
1. Loosen the screws of the battery
compartment lid with the screwdriver
supplied, remove the battery compart-
ment cover and insert the supplied NiMH
batteries into the battery compartment.
4. Insert the handheld into the charging
station as shown.
3. Connect the plug-in power supply unit
to the supply voltage. (Rated Voltage:
100-240V, Rated Current: 160-80mA)
CAUTION
Batteries inserted inversely can reach high temperatures and damage the de-•
vice.
18
Table of contents

















