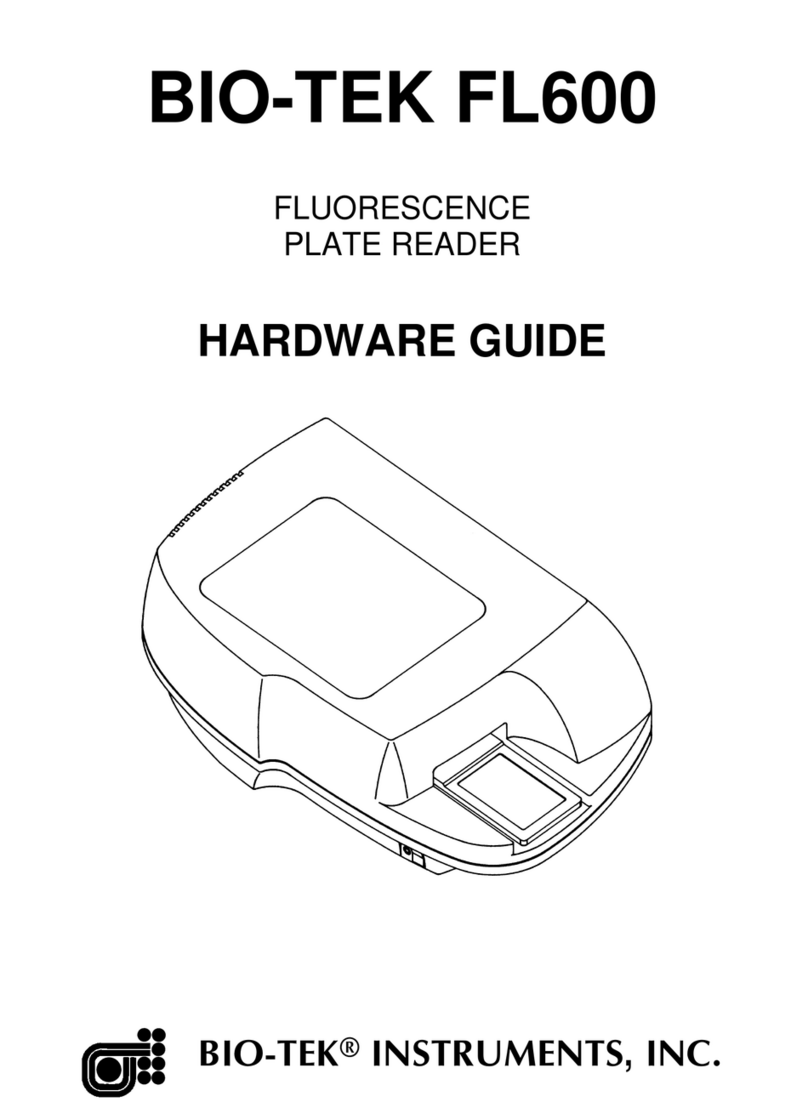
Bio-Tek Cytation 5 User manual

Cytation™ 5
Cell Imaging Multi-Mode Reader
Instructions for Use
BioTek®Instruments, Inc.
January 2015
© 2015
PN 1321022
Revision A

ii | Preface
Notices
BioTek®Instruments, Inc.
Highland Park, P.O. Box 998
Winooski, Vermont 05404-0998 USA
All Rights Reserved
© 2015, BioTek®Instruments, Incorporated. No part of this publication may be reproduced,
transcribed, or transmitted in any form, or by any means electronic or mechanical, including
photocopying and recording, for any purpose other than the purchaser’s use without written
permission of BioTek Instruments, Inc.
Trademarks
BioTek®is a registered trademark, and Cytation™, Gen5™, BioStack™, and Take3™ are
trademarks of BioTek Instruments, Inc. Glowell™ is a trademark of LUX Biotechnology, Ltd.
Harta™ is a trademark of Harta Instruments.
Microsoft®, Windows®, and Excel®are either registered trademarks or trademarks of Microsoft
Corporation in the United States and/or other countries.
All other trademarks are the property of their respective holders.
Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by
BioTek Instruments, Inc. Changes made to the information in this document will be incorporated
in new editions of the publication. No responsibility is assumed by BioTek for the use or
reliability of software or equipment that is not supplied by BioTek or its affiliated dealers.
BioTek Instruments, Inc.

Contact Information| iii
Contact Information
Customer Service and Sales
Internet:
www.biotek.com
Phone:
888-451-5171 (toll-free in the U.S.)
802-655-4740 (outside the U.S.)
Fax:
802-655-7941
Email:
customercare@biotek.com
Global Service and Support
BioTek instrument service and repair is available worldwide at one of BioTek's
International Service Centers and in the field at your location. For technical
assistance, contact the Technical Assistance Center (TAC) at BioTek US—World
Headquarters. To arrange for service or repair of your instrument, contact the office
nearest you.
BioTek US—World Headquarters
BioTek China—Beijing
Phone: (802)655-4740
Toll-Free: (888)451-5171
Service Toll-Free: (800)242-4685
Email: Custom[email protected]
Service Email: TAC@biotek.com
www.biotek.com
Phone: +86 (10) 85865569
Email: infochina@biotek.com
Website: www.biotekchina.com.cn
BioTek China—Shanghai
BioTek France
Phone: +86 (021)50435800
Email: infochina@biotek.com
Website: www.biotekchina.com.cn
Phone: +33 (3) 89206329
Email: info@biotek.fr
Website: www.biotek.fr
BioTek Germany—European
Coordination Center
BioTek India
Phone: +49 (0) 71369680
Email: [email protected]
Website: www.biotek.de
Phone: +91 (22) 66870046
Fax: +91 (22) 28759944
Email: biotek@biotek.in
Website: www.biotek.in
BioTek Japan
BioTek Singapore
Phone: +81(0)3 5812 8109
Email: infojapan@biotek.com
Website: www.biotek.com/ja
Phone: +65 65922100
Email: singapore@biotek.com
Website: www.biotek.com
Cytation 5

iv | Preface
BioTek South Korea
BioTek Switzerland
Phone: +82 (0) 2 5624740
Email: korea@biotek.com
Website:
www.biotekinstruments.co.kr
Phone: +41 (41) 2504060
Email: info@biotek.ch
Website: www.biotek.ch
BioTek Taiwan
BioTek United Kingdom (UK)
Phone: +886(2)26277725
Email: infotaiwan@biotek.com
Website: www.biotek.com
Email: CustomerCare@biotek.com
Website: www.biotek.uk.com
Instructions for Use Requirements
This document fulfills the basic needs of persons operating this device, according to
the requirements of the In Vitro Diagnostic Directive for "Instructions for Use." Some
of the device's higher-level functions and features, as well as certain detailed
maintenance and qualification routines, are described in the Cytation 5 Operator's
Manual.
Intended Use Statement
The Cytation 5 is a hybrid multi-mode microplate reader. The performance
characteristics of the data reduction software have not been established with any
laboratory diagnostic assay. The user must evaluate this instrument and PC-based
software in conjunction with their specific assay(s). This evaluation must include the
confirmation that performance characteristics for the specific assay(s) are met.
•If the instrument has an "IVD" label, it may be used for clinical and non-clinical
purposes, including research and development. If there is no such label, the
instrument may be used only for research and development or other non-
clinical purposes.
Quality Control
It is considered good laboratory practice to run laboratory samples according to
instructions and specific recommendations included in the assay package insert for
the test to be conducted. Failure to conduct Quality Control checks could result in
erroneous test data.
BioTek Instruments, Inc.

Warnings| v
Warnings
Operate the instrument on a level, stable surface away from excessive
humidity.
Bright sunlight or strong incandescent light can reduce the linear
performance range of the instrument.
Measurement values may be affected by extraneous particles (such as dust)
in the microplate wells. A clean work area is necessary to ensure accurate
readings.
When operated in a safe environment according to the instructions in this
document, there are no known hazards associated with the instrument.
However, the operator should be aware of certain situations that could result
in serious injury; these may vary depending on the instrument model. See
Hazards and Precautions.
Hazards
The following hazards are provided to help avoid injury:
Warning! Power Rating. The instrument’s power supply or power
cord must be connected to a power receptacle that provides voltage
and current within the specified rating for the system. Use of an
incompatible power receptacle may produce electrical shock and fire
hazards.
Warning! Electrical Grounding. Never use a plug adapter to connect
primary power to the external power supply. Use of an adapter
disconnects the utility ground, creating a severe shock hazard. Always
connect the power cord directly to an appropriate receptacle with a
functional ground.
Warning! Service. Only qualified technical personnel should perform
service procedures on internal components.
Warning! Accessories. Only accessories that meet the
manufacturer's specifications shall be used with the instrument.
Warning! Lubricants. Do not apply lubricants to the microplate
carrier or carrier track. Lubricant on the carrier mechanism or
components in the carrier compartment will attract dust and other
particles, which may obstruct the carrier path and cause the instrument
to produce an error.
Warning! The instrument with all available modules weighs up to 80
lbs. (36.3 kg). Use two people when lifting and carrying the
instrument.
Warning! Liquids. Avoid spilling liquids on the instrument; fluid
seepage into internal components creates a potential for shock hazard.
If a spill occurs while a p
rogram is running, abort the program and turn
off the instrument. Wipe up all spills immediately. Do not operate the
instrument if internal components have been exposed to fluid. Contact
BioTek TAC for assistance.
Cytation 5

vi | Preface
Warning! Unspecified Use. Failure to operate the equipment
according to the guidelines and safeguards specified in this manual
could result in a hazardous condition.
Warning! Software Quality Control. The operator must follow the
manufacturer’s assay package insert when modifying software
parameters and establishing reading methods. Failure to conduct
quality control checks could result in erroneous test data.
Warning! Reader Data Reduction Protocol. No limits are applied to
the raw measurement data. All information exported via computer
control must be thoroughly analyzed by the operator.
Warning! Internal Voltage. Always turn off the power switch and
unplug the power supply before cleaning the outer surface of the
instrument or removing its top case.
Warning! Potential Biohazards. Some assays or specimens may
pose a biohazard. This hazard is noted by the symbol shown here.
Adequate safety precautions should be taken as outlined in the assay’s
package insert. Always wear safety glasses and appropriate protective
equipment, such as chemical-resistant rubber gloves and apron.
Warning! LED Lights. Serious eye injury may occur if you stare
directly at the LED during operation of the light. This hazard is noted by
the symbol shown here.
Warning! Pinch Hazard. Some areas of the dispense module can
present pinch hazards when the instrument is operating. The module is
marked with the symbol shown here. Keep hands/fingers clear of these
areas when the instrument is operating.
Precautions
The following precautions are provided to help avoid damage to the instrument:
Caution: Service. The instrument should be serviced by BioTek-
authorized service personnel. Only qualified technical personnel should
perform service procedures on internal components.
Caution: Spare Parts. Only approved spare parts should be used for
maintenance. The use of unapproved spare parts and accessories may
result in a loss of warranty and potentially impair instrument performance
or cause damage to the instrument.
Caution: Environmental Conditions. Do not expose the system to
temperature extremes. For proper operation, ambient temperatures should
remain within the range listed in the Specifications chapter. Performance
may be adversely affected if temperatures fluctuate above or below this
range. Storage temperature limits are broader.
BioTek Instruments, Inc.

| vii
Caution: Sodium Hypochlorite. Do not expose any part of the
instrument to the recommended diluted sodium hypochlorite solution
(bleach) for more than 20 minutes. Prolonged contact may damage the
instrument surfaces. Be certain to rinse and thoroughly wipe all surfaces.
Caution: Power Supply. Use only the power supply shipped with the
instrument. Operate this power supply within the range of line voltages
listed on it.
Caution: Disposal. Dispose of the instrument according to Directive
2012/19/EC, “on waste electrical and electronic equipment (WEEE)” or
local ordinances.
Caution: Warranty. Failure to follow preventive maintenance protocols
may void the warranty. See the Maintenance chapter.
Caution: Shipping Hardware. The shipping brackets must be removed
before operating the instrument. They must be reinstalled before shipping
the instrument. See the Installation chapter.
Caution: Electromagnetic Environment. Per IEC 61326-2-6 it is the
user’s responsibility to ensure that a compatible electromagnetic
environment for this instrument is provided and maintained in order that
the device will perform as intended.
Caution: Electromagnetic Compatibility. Do not use this device in
close proximity to sources of strong electromagnetic radiation (e.g.,
unshielded intentional RF sources), because these may interfere with the
proper operation.
Cytation 5

viii | Preface
CE Mark
Based on the testing described below and information
contained herein, this instrument bears the CE mark
Refer to the Declaration of Conformity for specific details.
Directive 2014/30/EU: Electromagnetic Compatibility
Emissions—Class A
The system has been type-tested by an independent, accredited testing laboratory
and found to meet the requirements of EN 61326-1: Class A for Radiated Emissions
and Line Conducted Emissions.
Verification of compliance was conducted to the limits and methods of EN 55011 –
(CISPR 11) Class A. In a domestic environment it may cause radio interference, in
which case you may need to mitigate the interference.
Immunity
The system has been type-tested by an independent, accredited testing laboratory
and found to meet the requirements of EN 61326-1 and EN 61326-2-6 for Immunity.
Verification of compliance was conducted to the limits and methods of the following:
EN 61000-4-2, Electrostatic Discharge
EN 61000-4-3, Radiated EM Fields
EN 61000-4-4, Electrical Fast Transient/Burst
EN 61000-4-5, Surge Immunity
EN 61000-4-6, Conducted Disturbances from RFI
EN 61000-4-8, Power Frequency Magnetic Field Immunity Test
EN 61000-4-11, Voltage Dips, Short Interruptions and Variations
Directive 2014/35/EU Low Voltage (Safety)
The system has been type-tested by an independent testing laboratory and was
found to meet the requirements of this Directive. Verification of compliance was
conducted to the limits and methods of the following:
EN 61010-1. "Safety requirement for electrical equipment for measurement, control
and laboratory use. Part 1, General requirements."
EN 61010-2-081. “Particular requirements for automatic and semi-automatic
laboratory equipment for analysis and other purposes.”
EN 61010-2-010. “Particular requirements for laboratory equipment for the heating of
materials.“
BioTek Instruments, Inc.

Electromagnetic Interference and Susceptibility| ix
EN 60825-1, "Safety of laser products. Part 1: Equipment classification and
requirements."
Directive 2012/19/EU: Waste Electrical and Electronic
Equipment
Disposal Notice: Dispose of the instrument according to Directive 2002/96/EC, “on
waste electrical and electronic equipment (WEEE)” or local ordinances.
Directive 98/79/EC: In Vitro Diagnostics (if labeled for this
use)
•Product registration with competent authorities
•EN 61010-2-101. “Particular requirements for in vitro diagnostic (IVD) medical
equipment.”
•Traceability to the U.S. National Institute of Standards and Technology (NIST).
Electromagnetic Interference and Susceptibility
USA FCC CLASS A
RADIO AND TELEVISION INTERFERENCE
NOTE: This equipment has been tested and found to comply with the limits for a
Class A digital device, pursuant to Part 15 of the FCC Rules. These limits are
designed to provide reasonable protection against harmful interference when the
equipment is operated in a commercial environment. This equipment generates, uses,
and can radiate radio frequency energy and, if not installed and used in accordance
with the instruction manual, may cause harmful interference to radio
communications. Operation of this equipment in a residential area is likely to cause
harmful interference, in which case the user will be required to correct the
interference at their own expense.
In order to maintain compliance with FCC regulations, shielded cables must be used
with this equipment. Operation with non-approved equipment or unshielded cables
is likely to result in interference to radio and television reception.
Canadian Department of Communications Class A
This digital apparatus does not exceed Class A limits for radio emissions from digital
apparatus set out in the Radio Interference Regulations of the Canadians Department
of Communications.
Le present appareil numerique n'emet pas du bruits radioelectriques depassant les limites
applicables aux appareils numerique de la Class A prescrites dans le Reglement sur le
brouillage radioelectrique edicte par le ministere des Communications du Canada.
Cytation 5

x| Preface
User Safety
This device has been type-tested by an independent laboratory and found to meet the
requirements of the following:
•Underwriters Laboratories UL 61010-1, “Safety requirements for electrical
equipment for measurement, control and laboratory use; Part 1: General
requirements.”
•Canadian Standards Association CAN/CSA C22.2 No. 61010-1, “Safety
requirements for electrical equipment for measurement, control and laboratory
use; Part 1: General requirements.”
•EN 61010 Standards, see CE Mark starting on page viii.
BioTek Instruments, Inc.

Safety Symbols| xi
Safety Symbols
Some of the following symbols may appear on the instrument or accessories:
Alternating current
Courant alternatif
Wechselstrom
Corriente alterna
Corrente alternata
Warning, risk of crushing or
pinching
Attention, risque d'écrasement et
pincement
Warnen, Gefahr des Zerquetschens
und Klemmen
Precaución, riesgo del
machacamiento y sejeción
Attenzione, rischio di schiacciare ed
intrappolarsi
Direct current
Courant continu
Gleichstrom
Corriente continua
Corrente continua
Warning, hot surface
Attention, surface chaude
Vorsicht, heiße Oberfläche
Precaución, superficie caliente
Attenzione, superfice calda
Both direct and alternating
current
Courant continu et courant
alternatif
Gleich - und Wechselstrom
Corriente continua y
corriente alterna
Corrente continua e corrente
alternata
Laser radiation: Do not stare into
beam
Rayonnement laser: Ne pas
regarder dans le faisceau
Laserstrahlung: nicht in den strahl
blicken
Radiación de laser: No mire
fijamente al rayo
Radiazione di laser: Non stare nel
fascio
Earth ground terminal
Borne de terre
Erde (Betriebserde)
Borne de tierra
Terra (di funzionamento)
Warning, potential biohazards
Attention, risques biologiques
potentiels
Warnung! Moegliche biologische
Giftsoffe
Atención, riesgos biológicos
Attenziones, rischio biologico
Protective conductor
terminal
Borne de terre de protection
Schultzleiteranschluss
Borne de tierra de
protección
Terra di protezione
Caution (refer to accompanying
documents)
Attention (voir documents
d'accompanement)
Achtung siehe Begleitpapiere
Atención (vease los documentos
incluidos)
Attenzione, consultare la doc
annessa
Cytation 5

xii | Preface
On (Supply)
Marche (alimentation)
Ein (Verbindung mit dem
Netz)
Conectado
Chiuso
Consult instructions for use
Consulter la notice d'emploi
Gebrauchsanweisung beachten
Consultar las instrucciones de uso
Consultare le istruzioni per uso
Off (Supply)
Arrêt (alimentation)
Aus (Trennung vom Netz)
Desconectado
Aperto (sconnessione dalla
rete di alimentazione)
In vitro diagnostic medical device
Dispositif médical de diagnostic in
vitro
Medizinisches In-Vitro
Diagnostikum
Dispositivo médico de diagnóstico
in vitro
Dispositivo medico diagnostico in
vitro
Warning, risk of electric
shock
Attention, risque de choc
électrique
Gefährliche elektrische
schlag
Precaución, riesgo de
sacudida eléctrica
Attenzione, rischio di scossa
elettrica
Separate collection for electrical
and electronic equipment
Les équipements électriques et
électroniques font l'objet d'une
collecte sélective
Getrennte Sammlung von Elektro-
und Elektronikgeräten
Recogida selectiva de aparatos
eléctricos y electrónicos
Raccolta separata delle
apparecchiature elettriche ed
elettroniche
BioTek Instruments, Inc.

Installation

2| Installation
Package Contents
Item
Part #
Cytation 5 Operator's Manual (delivered on USB flash
drive)
1321000
Power cord set (specific to installation environment):
Europe (Schuko)
USA/International
United Kingdom
Australia/New Zealand
75010
75011
75012
75013
USB cable 75108
#2 Phillips screwdriver
01188
9/64" hex wrench
01623
Models with the imaging module:
FireWire desktop interface
OR
FireWire laptop card and power supply
01604
1220535
Power supply only:
01062
FireWire cable
1220538
Microplate slide holder
1220548
Isolation table
1220521
Objective adapter collar wrench
1222187
Objective setup plate
1222531
3/32" hex wrench
48570
Models with an external dispense module (packed separately), with the following
accessories:
Injector
8040541
Inlet tubes (2) from supply bottles to syringe drives
7082121
250-µL syringes (2)
7083000
BioTek Instruments, Inc.

1: Unpack and Inspect the Reader | 3
Item
Part #
Syringe thumbscrews
19511
Priming plate
8042202
Injector tip priming trough
8042068
Dispense module communication cable
75107
Dispense module front cover
8042197
Dispense module box
8040534
Supply bottles (2, 30 mL)
7122609
Supply bottle holders (2)
8042193
Injector tip cleaning stylus and plastic storage bag
2872304
Strap reagent racks (6)
7212035
Models with the gas controller ("G" models)(packed separately):
Gas controller unit, CO2/O2control
1210500
Shipping accessories, CO2/O2control
1210010
Gas Controller Unit, CO2only
1210504
Shipping accessories, CO2only
1210009
1: Unpack and Inspect the Reader
The Cytation 5 should be removed from the box by two people. The instrument
with all available modules weighs up to 80 pounds (36.6 kg).
Save all packaging materials. If you need to ship the reader to BioTek for repair
or replacement, you must use the original materials. Using other forms of
commercially available packaging, or failing to follow the repackaging
instructions, may void your warranty.
During the unpacking process, inspect the packaging, reader, and accessories
for shipping damage. If the reader is damaged, notify the carrier and your
BioTek representative. Keep the shipping boxes and the packaging materials
for the carrier's inspection. BioTek will arrange for repair or replacement
immediately.
1. Open the shipping box, remove the instrument from the box, and place it on
a level, stable surface.
2. Place the packaging materials back into the shipping box for reuse if the
instrument needs to be shipped again.
3. For the instruments with the imaging module: Open the accessories box, and
remove the isolation table.
Cytation 5

4| Installation
2: Unpack and Inspect the Dispenser
If applicable:
1. Open the shipping box. Remove the accessories box and foam insert that
contains the injector tubing and bottle holders.
2. Lift out the dispenser and place it on a level surface.
3. Open the accessories box and remove its contents.
4. Place all packaging materials into the shipping box for reuse if the dispenser
needs to be shipped.
3: Unpack and Inspect the Gas Controller
If applicable:
1. Open the shipping box. Remove the accessories, and set them aside.
2. Lift out the gas controller, and place it on a level surface.
3. Place all packaging materials into the shipping box for reuse if the gas
controller needs to be shipped.
4: Select an Appropriate Location
Install the reader on a level, stable surface in an area where ambient temperatures
between 18°C (64°F) and 30°C (86°F) can be maintained.
Leave at least six inches of space between the instrument’s rear panel and any other
object. This space ensures proper air flow in and out of the instrument.
The reader is sensitive to extreme environmental conditions. Avoid the following:
•Excessive humidity. Condensation directly on the sensitive electronic circuits
can cause the instrument to fail internal self-checks. The humidity must be in
the range of 10–85%, non-condensing.
•Excessive ambient light. Bright light may affect the reader’s optics and
readings, reducing its linear range.
•Dust. Readings may be affected by extraneous particles (such as dust) in the
microplate wells. A clean work area is necessary to ensure accurate readings.
•Vibration. The instrument should be installed in a vibration-free environment.
Be sure to position the instrument away from other devices that could
potentially create vibration during the read process.
If you are installing a BioStack for operation with the Cytation 5, you may
wish to seat the instruments in their alignment plates now. Refer to the
stacker's operator's manual for more information.
BioTek Instruments, Inc.

4: Select an Appropriate Location | 5
Installing Instruments with the Isolation Table
Cytation 5 models with the imaging module can be used with an isolation table,
which helps to eliminate vibration during image reads.
Do not use the isolation table when operating the Cytation 5 with a microplate
stacker. Store the isolation table in a clean, dry location.
1. Remove the four corner clips from the isolation table.
2. Place the isolation table in the selected installation location.
3. Place the instrument on the table as shown next:
Cytation 5

6| Installation
The isolation table contains material that dampens vibration. Over time, this material
becomes compressed and can lose effectiveness. The isolation table has a color
indicator that turns from green to red to show when the table should be replaced
because the dampening material has been compressed.
5: Remove the Shipping Hardware
Remove all shipping hardware before you turn on the reader.
1. Locate the shipping hardware.
The figures below depict a Cytation 5 with the filter module and imaging
module.
BioTek Instruments, Inc.

6: Install the Power Supply | 7
2. Open the top door, and remove the reusable zip tie and desiccant packet
from the desiccant anchor.
3. If equipped, use the supplied screwdriver to remove the top filter shipping
bracket.
4. Open the front door, and using the supplied screwdriver, remove the carrier
shipping bracket.
5. If equipped with the imaging module, use a 9/64" hex wrench to remove the
bottom filter slide ship bracket
6. Push the filter slide back, and remove the two-piece objective ship bracket.
7. Store the shipping hardware in a safe location, in case the instrument needs
to be shipped again.
6: Install the Power Supply
Power Rating. The instrument must be connected to a power receptacle that
provides voltage and current within the specified rating for the system. Use of
an incompatible power receptacle may produce electrical shock and fire
hazards.
Electrical Grounding. Never use a plug adapter to connect primary power to
the instrument. Use of an adapter disconnects the utility ground, creating a
severe shock hazard. Always connect the system power cord directly to an
appropriate receptacle with a functional ground.
1. Locate the power inlet on the back of the reader.
2. Examine the power supply's plug. It has a small groove that lines up with a
tab inside the power inlet.
3. Insert the plug into the power inlet and plug the power supply's cord into an
appropriate power receptacle.
Cytation 5

8| Installation
Do not plug the power supply into a power receptacle until after the
power supply is connected to the instrument.
7: Install the Gas Controller (if applicable)
The gas controller is an external module that enables the user to control CO2and O2
concentrations inside the attached instrument’s reading chamber. If you purchased
the module for operation with the Cytation 5, refer to the Gas Controller User Guide
for installation instructions.
8: Unpack and Install the Joystick (if
applicable)
If applicable:
1. Open the shipping box, lift out the joystick, and place it on a level surface.
2. Place all packaging materials into the shipping box for reuse if the joystick
needs to be shipped.
3. Locate the joystick cable. Plug one end into the port on the back of the
joystick. Plug the other end into the joystick port on the rear of the reader.
9: Install the Dispenser
Place the dispense module on top of the reader or on top of the gas
controller (if equipped). Do not place the dispenser next to the reader.
BioTek Instruments, Inc.
Table of contents
Other Bio-Tek Card Reader manuals