BIOLUX RESEARCH OrthoPulse User manual

Smile.
OrthoPulse®
User Guide


1
Contents
1. Introduction
1.1 About OrthoPulse®
1.2 Intended Use / Indications for Use
1.3 Contraindications for Use
2. How to Use
2.1 Steps and Schedule for Use
2.2 Charging
2.3 OrthoPulse®App
3. Care and Maintenance
3.1 Cleaning
3.2 Storage
3.3 Service Life
3.4 Replacement
3.5 Environmental Protection Disposal
4. Support
4.1 Orthodontic Treatment
4.2 Device Inquiries
4.3 Troubleshooting
4.4 Warranties
5. Safety
5.1 Technical Description and Classifications
5.2 Environmental Conditions
5.3 EMC Compliance Statement
5.4 Electromagnetic Compatibility
5.5 Power Adaptor Specification
5.6 Warnings and Safety Notices
2
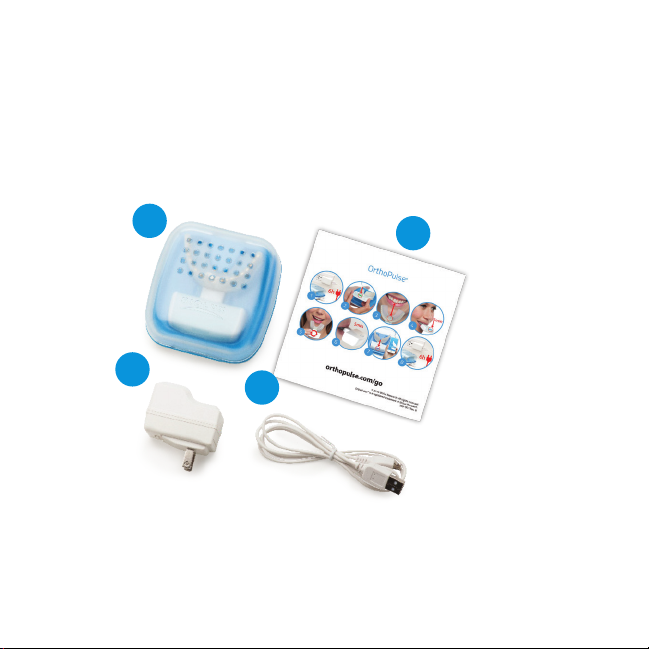
Inside Your OrthoPulse®Box
12
3
4

3
Ensure that all package contents are enclosed and that there is no
visible damage. Power adaptor may be slightly different than that
shown.
1 2
3 4
Charging Case Quick Start
Power Adaptor Micro USB Cord

4
English

55
1. Introduction
1.1 About OrthoPulse®
OrthoPulse®is an established device that uses
low levels of light energy to stimulate the bone
surrounding the roots of your teeth which may
reduce treatment time for braces or clear aligners.
OrthoPulse®uses low intensity near infra-red light
technology to gently facilitate orthodontic tooth
movement.
For further information about the clinical
benefits and supporting research, please visit
orthopulse.com
1.2 Intended Use / Indications for Use
The OrthoPulse®device is intended to accelerate
orthodontic movement of teeth and reduce
the overall treatment time for the patient. The
device is intended to be used in conjunction with
traditional orthodontic treatment with brackets
and wires or aligners.
OrthoPulse®is operated under prescription by
your orthodontist or dentist. Your prescribing
orthodontist or dentist is your best resource for
information regarding your orthodontic treatment
and the OrthoPulse®device.
Please direct questions regarding your orthodontic
treatment plan toward your prescribing
orthodontist or dentist. Biolux Research is not
authorized and unable to make representations
related to patient-specific treatment and/or
provide orthodontic treatment advice.
WARNING: OrthoPulse®is a single patient
prescription device. Do not use the OrthoPulse®
appliance on multiple patients. Use by an individual
without the proper issuance from an orthodontist
may result in unintended consequences, including
the possible transmission of viral and bacterial
infective agents.
United States and Hong Kong Indications
for Use
The OrthoPulse®device is intended for use during
orthodontic treatment. It is used in conjunction
with brackets and wires or aligners and helps
facilitate minor anterior tooth movement.
OrthoPulse®is operated under prescription by
your orthodontist or dentist. Your prescribing
orthodontist or dentist is your best resource for
information regarding your orthodontic treatment
and the OrthoPulse®device. Your orthodontist or
dentist should assess the fit of your orthodontic
appliance (aligners or brackets and wires) at
every follow up visit to ensure that your teeth
are progressing at an appropriate rate, including
assessments of pressure, pain, air gaps, etc., as
applicable.
Please direct questions regarding your orthodontic
treatment plan toward your prescribing
orthodontist or dentist. Biolux Research is not
authorized and unable to make representations
related to patient-specific treatment and/or
provide orthodontic treatment advice.
English

6
no serious adverse events, and no root resorption,
gingival recession or pathological tooth mobility
reported throughout the study.
OrthoPulse®was also evaluated in conjunction
with brackets and wires in a controlled study
of 33 subjects (mean age 25.0 years). Matched
controls (based on subjects’ age, initial crowding,
eligibility criteria) were retrospectively selected
before any data analysis of the OrthoPulse®
subjects. Eligibility criteria included requiring
that the subjects have permanent dentition,
mild to moderate crowding with no labiolingually
displaced teeth, Class I or Class II by 1/2 cusp or
less, good oral hygiene, and be non-smoking.
Subjects who were pregnant, enrolled in another
study, had periodontally involved teeth, used
bisphosphonates during the study or had spaces
between anterior teeth were excluded. There
were no differences between groups in terms of
gender, ethnicity, age, and initial crowding. The
rate of tooth movement was measured using the
change in Little’s Irregularity Index measurements
in both groups to evaluate OrthoPulse® use with
fixed orthodontic appliances. Root resorption was
determined by use of panoramic dental X-rays
collected before treatment and after 6 months of
treatment. Results demonstrated that subjects
treated with OrthoPulse®showed a statistically
signifigantly faster rate of tooth movement
(p<0.001) compared to the control group,
achieving the primary effectiveness objective of
the study. There were no serious adverse events,
and no gingival recession or pathological tooth
mobility reported throughout the study. Data
Clinical Evaluations of OrthoPulse®
Clinical testing of the OrthoPulse®device with
orthodontic treatment demonstrated that the
device may accelerate tooth movement and
may decrease treatment time. Two primary
clinical studies of the intra-oral OrthoPulse®
demonstrated device performance for its intended
use; the device may accelerate orthodontic
movement of teeth and may reduce the overall
treatment time for the patient when used in
conjunction with traditional orthodontic treatment
with brackets and wires or aligners.
In a cross-over study where subjects served as
their own control, 21 subjects (mean age 34.9
years) who used OrthoPulse®with aligners were
evaluated. Eligibility criteria included requiring
that the subjects have permanent dentition,
mild to moderate crowding with no labiolingually
displaced teeth, Class I or Class II by 1/2 cusp or
less, good oral hygiene, and be non-smoking.
Subjects who were pregnant, enrolled in another
study, had periodontally involved teeth, used
bisphosphonates during the study or had spaces
between anterior teeth were excluded. Perimeter
measurement analysis was used to evaluate each
patient’s rate of tooth movement during baseline
and OrthoPulse®periods in the mandibular arch.
The degree of external apical root resorption was
also investigated. Study subjects were followed
from the start of orthodontic aligner treatment
for 6 months. Results demonstrated statistically
significant faster tooth movement compared
to baseline (p=0.024), achieving the primary
effectiveness objective of the study. There were

7
2. How to Use
2.1 Steps and Schedule for Use
An OrthoPulse®treatment takes five minutes
per arch for a total of ten minutes daily. It is
recommended to select the same time everyday
to do your treatment.
The status light guide is available on the bottom of
the OrthoPulse®charging case.
Typically, it takes two to three weeks to develop
a habit, so be patient. Some patients prefer to
set up OrthoPulse®next to their bed, so they can
do treatments first thing upon rising or prior to
sleeping.
You may pause the treatment for up to 20 seconds
by simply removing the device from your mouth.
If you pause for more than 20 seconds, the
treatment will abort and you will have to restart
your OrthoPulse®treatment.
To use your OrthoPulse®, complete the six steps
below:
1. Remove the OrthoPulse®from the charging
case, this will wake the device from sleep mode.
The status light will display green upon waking
when the battery has sufficient charge to
complete a treatment. If a yellow light appears,
return it to the charging case.
2. Place the OrthoPulse®device in your mouth,
centering it between the front teeth.
demonstrated the absence of external apical root
resorption with OrthoPulse®use, and that there is
no device effect of accelerated tooth movement
on tooth root integrity.
Several additional clinical studies were also
conducted with prototype and final OrthoPulse®
devices to supplement the clinical findings
observed in the primary studies, and results
consistently confirmed device performance for its
indicated use.
Therefore, results from the clinical studies
demonstrate that subjects treated with
OrthoPulse®achieve statistically significantly
faster rates of tooth movement than control.
The amount of change in an individual’s tooth
movement rate during OrthoPulse®daily
treatment may be dependent upon their specific
biology and treatment plan. For clear aligners, only
Invisalign brand aligners have been examined with
daily OrthoPulse®use. Results with other brands
of aligners may vary.
1.3 Contraindications for Use
•Use of osteoporosis drugs
•Use of drugs that may cause photosensitivity
•Photosensitivity
•Poor oral hygiene
•Acute oral infection, active periodontal disease
or oral cancer
•Photosensitive epilepsy
A dental professional should be consulted prior to
use if any of these situations are suspected.

8
CAUTION: Place the OrthoPulse®on a stable
flat surface and out of the way to avoid tripping
hazards.
2.3 OrthoPulse®App
Biolux has developed an app to help patients
and doctors follow their OrthoPulse®treatment
compliance, stay motivated, and achieve great
smiles faster.
The app is compatible with iOS and Android
products.
iOS Compatibility: Requires iOS 10.0 or higher.
Android Compatibility: Devices running Android 5.0
or higher with Bluetooth®LE (4.0).
In order to use the OrthoPulse®app, your
orthodontist or dentist must first create a patient
account for you. As soon as they do so, you will
receive a welcome email with your username and
password to log into the app. If you do not receive
your welcome email, make sure to check your
To install the OrthoPulse®app, use the download
link provided in your welcome email or go to the
Apple App Store or Google Play Store and type
“orthopulse” in the search field. Use the login
credentials provided to you in the welcome email.
Upon logging in, the app will start searching for
your OrthoPulse®device with which to sync. In
order for the sync to take place, both your mobile
device and OrthoPulse®must be in Bluetooth®
mode, and there must be a stable internet
connection. To activate Bluetooth®mode on your
3. Bite down gently to hold it in place. The device
will beep twice and the status light will turn blue
indicating that the treatment has started. A warm,
pleasant sensation can be felt during treatment.
4. Once the treatment is complete, the device will
beep three times continuously and the blue status
light will start pulsing.
5. Flip the device and repeat steps 2 through 4 to
treat the other jaw.
6. Return the device to the plugged-in charging
case to re-charge the device after treatment.
TIP: Avoid loud background noise during treatment
to ensure you hear the aural indicators.
2.2 Charging
Using the micro-USB cable, connect the charging
case to the power adaptor and plug it into a power
outlet to charge the device.
Approximately three hours are needed to fully
recharge the OrthoPulse®device. A green status
light will indicate a sufficient battery charge to
complete two treatment sessions. When the
device is fully charged, the status light will turn off
and the device will sleep automatically.
Two sessions can be completed on one full
charge. The device must be recharged after each
10-minute treatment. If the status light is solid
yellow, the device needs to be recharged prior to
use.

9
dry place away from direct sunlight. Avoid storing
your OrthoPulse®in locations where it may be
exposed to extreme temperatures.
CAUTION: The OrthoPulse®should be stored out of
the reach of young children or pets; it is not a toy.
3.3 Service Life
OrthoPulse®should last for the duration of your
orthodontic treatment. The device should last for
up to two years of continuous use if used with
care.
The OrthoPulse®device contains a lithium
polymer battery that will lose charge over time
if not re-charged. The OrthoPulse®device should
be fully charged within three months of delivery
and should be fully charged prior to first use.
To maintain battery life, do not let the battery
completely discharge.
3.4 Replacement
No component of the OrthoPulse®device is user-
serviceable or -replaceable. During the course
of treatment, no OrthoPulse®components
should require replacement. Bite marks and
other wear marks that become present in the
mouthpiece over time are normal, and do not
require replacement. However, they may be
indications that you are biting or clenching too
hard during your OrthoPulse®treatment. If there
are punctures, or any of the internal surfaces
of the mouthpiece become exposed, stop using
the OrthoPulse®immediately and contact
OrthoPulse®, pick it up and place it back down on
the charging case so that the status light displays
purple.
If no communication is achieved between
OrthoPulse®and the app within 60 seconds,
Bluetooth®will time out after 60 seconds and you
will have to reactivate Bluetooth®mode on your
OrthoPulse®.
3. Care and Maintenance
3.1 Cleaning
It is not necessary to clean OrthoPulse®after
every use. It is recommend that patients rinse the
mouthpiece under warm water once a week and
set it to air dry on the charging case.
Hold the OrthoPulse®device by the white plastic
housing – do not hold it by the silicone mouthpiece.
CAUTION: The OrthoPulse®device is NOT
dishwasher safe.
CAUTION: The charging case is not water resistant
and should not be rinsed or submerged in water.
The charging case should be used in a dry
environment, inside, and kept away from water.
3.2 Storage
Store your OrthoPulse®in its charging case when
not in use. Use the sliding lock to secure the device,
particularly when travelling. This will prevent
damage.
The OrthoPulse®device should be stored in a cool,

10
4. Support
4.1 Orthodontic Treatment
Please contact your orthodontist or dentist
directly for all inquires regarding your treatment.
4.2 Device Inquiries
Please contact the OrthoPulse®Support Team:
•for assistance in setting up, using or maintaining
your OrthoPulse®
•to report unexpected operation or events
•for technical assistance and any concerns
specifically related to OrthoPulse®or its
accessories
Manufacturer Contact Information:
Biolux Research Holdings Inc.
47669 Fremont Blvd.
Fremont CA, 94538 USA
Email: [email protected]
Web: orthopulse.com
Patented orthopulse.com/patents
4.3 Troubleshooting
Visit the FAQ section on the OrthoPulse®website,
available here:
http://www.orthopulse.com/patients/support
4.4 Warranties
Limited Warranty: Biolux Research (Biolux)
warrants to the original purchaser that the
OrthoPulse®device will be free from defects in
material and workmanship for one (1) year from
the date of the original purchase from Biolux or
In case of other damage or unforeseen wear and
WARNING: Do not tamper with or attempt to
repair your OrthoPulse®or its charging case.
If your OrthoPulse®becomes damaged, contact
repair. Prior to use, inspect OrthoPulse®for
noticeable signs of damage or wear. Do not
substitute any parts or materials in the device.
3.5 Environmental Protection Disposal
The user guide and packaging are recyclable and
should be disposed of with other recyclable paper
products. To preserve the environment and protect
human health, the device should not be disposed
of with normal household waste.
Dispose of your device, charging case, micro-USB
cable and power supply by delivering them to a
designated collection point for the recycling of
waste electrical and electronic equipment.
WARNING: Never incinerate OrthoPulse®, expose
to excessive heat, short circuit or cause any similar
action to the battery. Mishandling the battery may
cause burns, fire or explosion.
Contact your local waste authorities,
your household waste disposal service or
information regarding disposal.

11
FOR A PARTICULAR PURPOSE, PERFORMANCE,
QUALITY, OR DURABILITY, ALL OF WHICH ARE
DISCLAIMED. IN NO EVENT WILL BIOLUX BE
LIABLE FOR ANY SPECIAL, EXTRAORDINARY,
INDIRECT OR CONSEQUENTIAL DAMAGES OF
ANY KIND WHATSOEVER, INCLUDING WITHOUT
LIMITATION DAMAGES FOR LOSS OF DATA, LOST
PROFITS, LOSS OF OPPORTUNITY, BUSINESS
INTERRUPTION, PERSONAL INJURY OR DEATH,
OR ANY OTHER LOSS ARISING OUT OF, RELATING
TO, OR IN CONNECTION WITH THE ORTHOPULSE®,
EVEN IF BIOLUX IS ADVISED OF THE POSSIBILITY
OF SUCH DAMAGES. LIABILITY LIMITATIONS: IF,
AS A RESULT OF OR IN CONNECTION WITH ANY
USE OF THE ORTHOPULSE®, BIOLUX BECOMES
LIABLE TO THE PURCHASER OR ANY OTHER
PERSON FOR ANY DAMAGES, LOSSES, COSTS,
EXPENSES, OR OTHER LIABILITIES WHATSOEVER,
AND REGARDLESS OF THE FORM OF ACTION (IN
CONTRACT, TORT OR PURSUANT TO STATUTE),
THEN BIOLUX’S AGGREGATE LIABILITY WILL
BE LIMITED TO AN AMOUNT EQUAL TO THE
PURCHASE PRICE PAID FOR THE ORTHOPULSE®.
The exclusion of certain conditions and warranties
and time limitation of certain liability is prohibited
in some jurisdiction, so these limitations and
exclusions may not apply to some purchasers.
This limited warranty is governed solely by the
laws of the Province of British Columbia, Canada
and applicable federal laws of Canada, excluding
any rules of private international law or the conflict
of laws which would lead to the application of any
other laws; the courts of British Columbia, Canada
shall have exclusive jurisdiction over any claims
its authorized resellers. This limited warranty is
non-transferrable. If the OrthoPulse® is defective
during the warranty period, the purchaser’s
sole and exclusive remedy, and Biolux’s sole
obligation, will be (at Biolux’s discretion) to: repair
the OrthoPulse®to conform to its specifications;
replace the OrthoPulse®with a comparable
product; or refund to the purchaser the original
price paid for the OrthoPulse®. Repaired or replaced
products or parts may be new or reconditioned,
and are subject to this limited warranty through
the end of the original warranty period. To obtain
warranty service, the purchaser must: contact the
prescribing orthodontist or dentist. This warranty
does not apply if the defect or malfunction in
the OrthoPulse®was caused by misuse, neglect,
unauthorized attempts to open, repair or modify
the OrthoPulse®, use of the OrthoPulse®with
accessories or other products that are not
authorized by Biolux, or any cause other than
the intended normal use of the OrthoPulse®.
Non-warranty work is charged at the minimum
repair rate effective at the time the OrthoPulse®is
returned to Biolux. All repairs include a complete
functional test using factory test fixtures.
EXCLUSIONS: TO THE FULL EXTENT ALLOWED
BY LAW, THIS LIMITED WARRANTY IS THE
PURCHASER’S SOLE AND EXCLUSIVE REMEDY,
AND NO OTHER WARRANTIES, CONDITIONS,
OR GUARANTEES OF ANY KINDS SHALL APPLY,
WHETHER STATUTORY, WRITTEN, ORALLY
EXPRESSED OR IMPLIED; INCLUDING WITHOUT
LIMITATION WARRANTIES, CONDITIONS OR
GUARANTEES OF MERCHANTABILITY, FITNESS

12
•Protection Class: Class II equipment.
Ingress Protection Class:
•OrthoPulse®is rated as IP37, is tool proof and
submersible in water up to 1 m deep for up
to 30 minutes.
•Charging case is rated as IP32, is tool proof and
resistant to dripping water while tilted 15°.
5.2 Environmental Conditions
Environmental Operating Conditions:
•Ambient temperature range: 5°C to 35°C
•Relative humidity range: 15 to 93% non-
condensing
•Atmospheric pressure range: 700 to 1060 hPa
Transport and Storage Conditions:
•Minimum ambient temperature: -20°C
•Maximum ambient temperature: 65°C
•Maximum humidity: 93% non-condensing
5.3 EMC Compliance Statement
This device has been tested and found to comply
with the limits for a Class B digital device, pursuant
to part 15 of the FCC rules. These limits are
designed to provide reasonable protection against
harmful interference in a residential setting.
This device generates, uses and can radiate radio-
frequency energy and, if not installed and used
in accordance with the instructions, may cause
harmful interference to radio communications.
However, there is no guarantee that interference
will not occur in a particular installation. If this
relating to this limited warranty.
Biolux Research Ltd. has US and international
patents pending for OrthoPulse®and the
accompanying technology.
The Biolux logo, OrthoPulse®, Light Accelerated
Orthodontics™, Great Smiles Faster™ and the
collection of these marks are trademarks of Biolux
Research. All rights reserved.
Manufacturers Liability
Biolux Research assumes no responsibility for
any damage, loss, or claims which may result
from: failure to follow the instructions contained
in this manual; malfunction due to unauthorized
repairs or modifications. Use of the OrthoPulse®
equipment is entirely the responsibility of the
operator.
5. Safety
5.1 Technical Description and
Classifications
The following is a technical description of
OrthoPulse®. It is intended to provide all data
essential for safe operation, transport and storage
as well as permissible environmental conditions
and electrical safety classifications.
WARNING: No modification or servicing of this
equipment is allowed.
•OrthoPulse® is considered to be an applied part
according to the IEC 60601-1 3rd Ed. OrthoPulse®
is classified as a Type BF applied part.

13
Transmitter Module Certifications
CE: Complies with Radio Equipment Directive, RED
2014/53/EU
FCC Limited Modular Certification 15.212 FCC
#2AAQS-ISP091201
Canada: IC # 11306A-ISP091201
Bluetooth SIG certified #B017595
USA - User Information
OrthoPulse®contains transmitter module FCC ID:
2AAQS-ISP091201.
This device complies with part 15 of the FCC
rules. Operation is subject to the following two
conditions: (1) This device may not cause harmful
interference, and (2) This device must accept any
interference received, including interference that
may cause undesired operation.
Caution: Any changes or modifications not
expressly approved by the party responsible for
compliance could void the user’s authority to
operate the equipment.
Canada - User Information
OrthoPulse® contains transmitter module IC ID:
11306A-ISP091201.
This device complies with Industry Canada licence-
exempt RSS standard(s). Operation is subject to
the following two conditions: (1) this device may
not cause interference, and (2) this device must
accept any interference, including interference
that may cause undesired operation of the device.
device does cause harmful interference to radio
or television reception, which can be determined
by turning the device off and on, the user is
encouraged to try one or more of the following
measures:
•Reorient or relocate the device or the receiver
•Increase the distance between the device and
the receiver
•Connect the device to an outlet on a circuit
different from that to which the receiver is
connected
•Consult the manufacturer or an experienced
broadcast engineer/ technician for help
Be aware that portable and mobile radio-frequency
communications equipment (for example, mobile
phones, iPads) may affect the operation of this
device; take appropriate precautions during
operation.
Accessories
To maintain electromagnetic compatibility (EMC)
within limits, the device must be used with the
cables and accessories specified by Biolux. The use
of accessories or cables other than those specified
or supplied may result in increased emissions or
decreased immunity of the device.
Radio-Frequency Transmitter
OrthoPulse®contains a Bluetooth LE transmitter
module that operates at 2.4 GHz. This module
is active only when the device is placed in the
charging case and the Ready for Bluetooth
indicator is on.

14
5.4 Electromagnetic Compatibility
This device is intended for use in a HOME HEALTHCARE ENVIRONMENT.
This device emits energy in the infrared range for a predetermined duration.
This device includes a micro-USB cable with a maximum length of 4’ 4” or 132 centimeters.
WARNING: Use of other accessories including power supplies and cables other than the ones provided
by Biolux Research for this device may result in increased electromagnetic emissions or decreased
electromagnetic immunity of this device and result in improper operation.
WARNING: Portable RF communications equipment (including peripherals such as antenna cables and
external antennas) should be used no closer than 30 cm (12 inches) to any part of this device, including
cables provided by Biolux Research. Otherwise, degradation of the performance of this equipment could
result.
Electromagnetic Emissions
Test or
Measurement
Standards Test Method Description Results
Radiated Emissions EN 60601-1-2:2015
Ed. 4
CISPR 11
EN 60601-1-11
EN 60601-2-57
EN 301 489-1
V2.1.1
ICES-003 Issue 6
CFR Title 47 FCC
Part 15
ICES-003 Issu.6
Class B Limits
The radiated
emissions are
measured in the
30-1000MHz
range or up to 5x
the highest EUT
frequency whichever
is higher*
Complies
Conducted
Emissions
The Conducted
Emissions are
measured on the
phase and Neutral
Power lines in the
0.15 - 30.0 MHz
range.

15
Test or
Measurement
Standards Test Method Description Results
*Highest frequency generated by the device is 2.4GHz
Emission Test Compliance Comments
RF Emissions
CISPR 11
Group 1 This device uses RF energy
only for its internal functions.
RF Emissions
CISPR 11
Class B This device is predominantly intended
for use in a HOME HEALTHCARE
ENVIROUNMNET
and to be connected to the PUBLIC
MAINS NETWORK
Harmonic Emissions
EN 61000-3-2
Class A
Voltage Fluctuations/
Flicker Emissions
EN 61000-3-3
Class A

16
Electromagnetic Immunity
Immunity Test Standard/Test Method Test Levels Compliance
Electrostatic Discharge IEC 61000-4-2 Air Discharge: ± 2, 4,
8, 15 kV
Contact Discharge: ± 8 kV
Complies
Radiated RF IEC 61000-4-3 10V/m, 80% AM @
1kHz, 30MHz to 2.5GHz,
Vertical and Horizontal
Polarizations
Complies
Immunity to Proximity
Fields from RF Wireless
Communications
Equipment
IEC 61000-4-3 9 V/m to 28 V/m @ 15
Frequencies 380 - 5800
MHz
Complies
Electrical Fast Transient/
Burst
IEC 61000-4-4 AC Power Lines: ± 2 kV @
100 kHz
Signal Lines: ± 1 kV @
100 kHz
Complies
Surge IEC 61000-4-5 ±0.5, 1 kV line to line, 0°,
90°,180°, 270°
±0.5. 1, 2 kV line to earth,
0°, 90°,180°, 270°
Complies
Conducted RF IEC 61000-4-6 3Vrms, 0.15-80MHz, 80%
AM @ 1 kHz
6Vrms in ISM & Amateur
radio bands, 0.15-80MHz,
80% AM @ 1 kHz
Complies
Power Frequency Magnetic
Field
IEC 61000-4-8 30 A/m Complies

17
Immunity Test Standard/Test Method Test Levels Compliance
Voltage Dips IEC 61000-4-11 0 % UT; 0,5 cycle at 0°,
45°, 90°, 135°, 180°,
225°, 270° and 315°
0 % UT; 1 cycle
70 % UT; 25/30 cycles
Complies
Voltage Interruptions IEC 61000-4-11 0 % UT; 250/300 cycles Complies
NOTE: UT is the AC mains voltage prior to application of the test level.
5.5 Power Adapter Specification
The supplied power adapter is part of the OrthoPulse®equipment and should be used to charge your
device.
The technical information for the power adapter is listed below:
Supplier: GlobTek Inc
Part number: WR9QA1200USBNMEDRVB
Model: GTM41078-0605-USB
Universal Input:
•Input Voltage: 100 ~ 240 VAC
•Input Frequency: 50 ~ 60 Hz
•Input Current: 0.3 A RMS max
Output Voltage: 5V
Maximum Output Current: 1.2 A
CAUTION: Only use the recommended micro-USB cable and AC adapter plug to charge your device.
5.6 Warnings and Safety Notices
United States Federal law and other national regulations restrict this device to sale by or on the order of
a doctor. Biolux Research Ltd. cannot be held responsible for any damage or injury resulting from a failure
to follow the directions in this user guide. Ensure that you are entirely familiar with the correct procedures
for operating the appliance before use.

18
Biolux Research (“we,” “our,” or “us”), collects,
uses, and discloses information about you through
the OrthoPulse®and the associated OrthoPulse®
mobile application (the “OrthoPulse®App”). (We
refer to the OrthoPulse®and the OrthoPulse®
App collectively as the “OrthoPulse®System”.) For
using an OrthoPulse® or the OrthoPulse®App,
you have to consent to the processing of your
information as set forth in this Privacy Policy,
now and as amended by us. Your use of www.
orthopulse.com, io.bioluxresearch.com and www.
bioluxresearch.com or OrthoPulse®Connect™ is
governed by a separate privacy policy, which is
available here: http://orthopulse.com/privacy-
policy and here, for patients under the age of
majority who require guardian/parental consent:
https://io.bioluxresearch.com/admin/doctor/
consent/exampleassent/
What Information Do We Collect?
The information we collect from users is an
essential component of the OrthoPulse®System:
Information You or Your Dental Provider Share with
Us: We and our service providers collect and store
any information that you provide to us, as well as
information that is provided to us by your dentist,
orthodontist or other treatment provider. If you,
your dentist, orthodontist or other treatment
provider create a provider or patient account
linked to your name or contact information (an
“Account”), we collect the registration information
that is shared with us. We collect information
when you contact us via the OrthoPulse®App
with a request, question, or comment. We collect
information about patients when dental providers
ATTENTION:
•Use only as directed. OrthoPulse®must be
used under the direction or supervision of an
orthodontist or dentist.
•Discontinue use if you have an allergic reaction
to OrthoPulse®or its accessories and seek
medical opinion.
•Chewing or clenching on the bite pad may
damage the device, or lead to a choking hazard.
During use, bite gently on the bite pad.
•Staring at the near-infrared light source may
cause eye irritation. Do not stare directly at the
mouthpiece.
•Avoid knocking, hitting or pulling your
OrthoPulse®with force. Rough handling may
cause damage. Discontinue use if damage is
suspected.
•The charging case and cable may be a tripping
hazard. Plug in near the wall outlet on a stable
flat surface.
•Do not use the device while operating machinery
or performing complex tasks.
•Do not use with high frequency (HF) surgical
equipment.
•Patients with an implanted cardiac pacemaker,
defibrillator, or an equivalent cardiac device
should not use OrthoPulse®unless the cardiac
device is known to not be affected by magnetic
fields.
MOBILE APP AND ORTHOPULSE®DEVICE
PRIVACY POLICY
This Privacy Policy describes the ways in which
Table of contents
Popular Personal Care Product manuals by other brands

Home Skinovations
Home Skinovations Silk'n Jewel manual

cliMATE
cliMATE CLI-AD-LED Installation & operating instructions

Silvercrest
Silvercrest SHK 100 B2 Operation and safety notes

Silk'n
Silk'n FaceFX DO105625B Instructions for use

Silk'n
Silk'n Infinity Instructions for use

Silvercrest
Silvercrest SRNH 100 D3 Operating instructions and safety advice

Silk'n
Silk'n Silhouette Instructions for use

Mastercare
Mastercare MC 001-1025 user manual

Silk'n
Silk'n VITALSTEAM NV8388 user manual

dj ortho
dj ortho Surround Ankle Stirrup w/Floam Application Instructions

KENT
KENT 12011 instruction manual
Otto Bock
Otto Bock Myoelectric Forearm Prosthesis Instructions for use





