BioSignals AI-HRV User manual

BioSignals LIMITED. All Rights Reserved. Page 1 of 19
AI–HRV User Manual
MADE BY BioSignals LIMITED

BioSignals LIMITED. All Rights Reserved. Page 2 of 19
User Manual
Note
Thanks very much for purchasing the AI -HRV produced by BioSignals LIMITED.
Read this manual carefully for correct operation before using the pulse oximeter.
Keep the manual properly for reference at any time when necessary.
Product Name
AI-HRV
Model
2022 – VER 1.0
Product structure and
component
The device consists of probe, electronic circuits, OLED/LED display (differentiated by
models) and plastic housing which powered by two alkaline AAA batteries.
Indication for use
The AI – HRV is indicated for the noninvasive spot checking of functional blood volume
pulse and saturation of arterial hemoglobin and pulse rate (PR) for adult and pediatric
(≥10Kg) customers during both no motion and motion conditions, and for customers who
are well.
Manufacturer
BioSignals LIMITED and it’s suppliers
Registration Address
UNIT 1, 1/F MAU LAM COMM BLDG 16-18 MAU LAM STREET, JORDAN,
KOWLOON HONG KONG
Website Address
www.BioSignals.co.il

BioSignals LIMITED. All Rights Reserved. Page 3 of 19
After-Sale Service
BioSignals LIMITED. (Hereinafter referred to as “the company”) shall assume responsibilities for the
return, replacement, and repair of the equipment in case of quality problems caused by non-human factors
within one week from the date of sale. The company will provide maintenance free of any charge for
quality problems occurring during normal use and storage within one year from date of production; If the
equipment has quality problems after one year from the date of production, users may carry invoice and
warranty card to our after-sale service department or agency and the company will provide spare parts for
maintenance with reasonable charge. The warranty period will be one month since the date of production
if users fail to provide invoice.
Warranty does not apply under the following circumstances:
A. Failure caused by disassembling, repairing or transforming the equipment without authorization.
B. Malfunction caused when the product falls carelessly during the course of use and transportation.
C. Damage caused by improper use.
D. Malfunction caused by operation not complying with the manual.
E. Damage caused by unforeseen natural disasters (earthquake, flood, etc.).
Table 1 After-Sale Service Information
After-sales service Unit
BioSignals LIMITED.
Registration Address
UNIT 1, 1/F MAU LAM COMM BLDG 16-18 MAU LAM STREET, JORDAN,
KOWLOON HONG KONG
Production Address
UNIT 1, 1/F MAU LAM COMM BLDG 16-18 MAU LAM STREET, JORDAN,
KOWLOON HONG KONG
Website
www.BioSignals.co.il

BioSignals LIMITED. All Rights Reserved. Page 4 of 19
1 Safety
1.1 Safety Information
1.1.1 Description
1. functional tester (for example: Fluke Index II, Smarts Set, ProSim8, etc) are only suitable for Blood
Volume Pulse waves accuracy test, and could not be used for any other performance test.
2. This AI -HRV does not provide an alarm function. User can get hinds on whether physiological signal
disturbed by refer to Section 7 Specification.
3. The working temperature of the AI -HRV is no more than 41℃. The test method is as follows: put NTC
resister between light emitter and finger, in order to get regional tissue’s temperature. The temperature
should not exceed to 41℃ while under 35℃ environments. User should change measure position regularly
to avoid temperature accumulative effect if take long-term measurement state. It might get harmful burns
if keeps long-term using under 37℃.
4. The performance of the AI -HRV is guaranteed by blood gas analysis which accord with International
Standard ISO80601-2-61 and YY0784. By performing hypoxia state in the range of 70%~100% SpO2 on
healthy adult male and female volunteers with different skin colors, it was confirmed that the saturation
measurements performance of AI -HRV meets the accuracy claim.
5. There is no need to re-calibrate because the AI -HRV has already calibrated. User can use Fluke Index
II functional tester to validate the function of the pulse oximeter during maintaining. Please contact
manufacturer to get the validate curve type for the functional tester.
1.1.2 Warning
1. Only qualified personnel designated by the manufacturer is allowed to maintain the equipment. Users
themselves are not allowed to repair the equipment.
2. Explosion hazard: not use the equipment in environment with inflammable substances such as
anesthetics.
3. Not use the equipment in MRI and CT examination.
4. When used with electrosurgical equipment together, the user shall ensure the safety of the monitored
patient.
5. Users who are allergic to silicone rubber is forbidden to use this equipment.
6. The equipment, accessories, and packaging (batteries, plastic bags, foam and cartons, etc.) shall be
scrapped in compliance with local laws and regulations.
7. Forbid to use the equipment once the equipment is found damaged or have material deterioration.
8. Do not use the equipment under the application conditions beyond its declared specification scope.
9. Check the sensor site every hour to ensure adequate circulation, skin integrity, and sensor alignment.
Skin damage, pressure necrosis, or inaccurate readings may result.
10.Do not use AI -HRV for continuous monitoring. It is intended for spot-check use only. No alarms are
provided to support continuous monitoring.
11.Misapplication of continuous monitoring with excessive pressure for prolonged periods might induce

BioSignals LIMITED. All Rights Reserved. Page 5 of 19
pressure injury.
12. Keep the device away from electrical equipment that emits radio frequencies to minimize radio
interference, such as diathermy, electrocautery, RFID and security systems (e.g., electromagnetic anti-theft
systems, and metal detectors). Radio interference may result in no or inaccurate readings.
1.1.3 Note
1. Take out batteries if the equipment is not used for a long time.
2. Performance of the equipment will be affected by electromagnetic field and therefore instruments
used near the AI -HRV shall meet EMC requirements. Mobile phones, X-ray or MRI equipment may
produce electromagnetic radiation interference.
3. Properly carry the equipment and prevent it from falling, collision, strong vibration or other
mechanical external force damage.
1.1.4 Caution
1. The equipment is only used to assist BVP and HRV diagnosis and the measurement data shall not be
directly applied in any clinical diagnosis.
2. It is not recommended to use the equipment in high frequency interference environment (eg. electric
knife).
3. Children are not recommended to operate the equipment directly and they must operate the equipment
under adult supervision.
4. Keep the application environment free of dust, vibration, corrosion or combustible substances, and avoid
temperature and humidity too high or too low.
5. Turn off the equipment immediately if water splash the equipment or there is condensate in the
equipment.
6. Do not use the equipment immediately when it is transferred from cold environment to warm and humid
place.
7. Incorrect battery installation will damage equipment, please check the polarity mark in the battery
housing when installing the battery.
8. There is a visual indicator of low battery capacity in the equipment. Replace batteries in time when the
low battery indicator appears.
9. When the AI -HRV is transferred from one place to another, the difference of temperature or humidity
may lead to the condensation phenomenon. At this time, user must wait for the condensation to disappear
before starting the AI -HRV.

BioSignals LIMITED. All Rights Reserved. Page 6 of 19
1.2 Symbol of Equipment
There are all or partial symbols in the equipment provided to you.
Symbol
Implication
Symbol
Implication
BF application part
No SpO2 alarm indication
(The equipment has no SpO2 alarm
function)
Batch number
Note: refer to attached documents
IP22
Degrees of protection provided by
enclosures
Date of manufacture
Fragile
Manufacturer information
No rain
This side up
Range of storage temperature
Stacking level
Range of storage humidity
Range of atmospheric pressure
--
Invalid measurement
Internal quantity
Dispose of in accordance to your
country’s requirements
Authorized representative in the European
Community
This is medical device
Caution: Federal law prohibits dispensing
without prescription.
Part number
Direct current
The product bears CE mark indicating
its conformity with Medical Device
Regulation (MDR) and fulfils the
essential requirements of Annex I of
this Regulation.

BioSignals LIMITED. All Rights Reserved. Page 7 of 19
2 Overview
2.1 Product Introduction
2.1.1 Measurement Principle
AI-HRV has red and infrared low voltage light emitting diodes (LEDs) which serve as light sources. The
emitted light is transmitted through the tissue, then detected by the photodetector and sent to the
microprocessor of the AI-HRV.
The pulsating of arterial blood results in changes in the absorption to added hemoglobin (Hb) and
oxygenated hemoglobin (HbO2) in the path of the light. Since HbO2 and Hb absorb light to varying
degrees, this varying absorption is translated into plethysmography waveforms at both red and infrared
wavelengths. The relationship of red and infrared plethysmography signal amplitude can be directly
related to arterial oxygen saturation by using Beer–Lambert law.
Functional Oxygen
Saturation (SpO2)
Functional oxygen saturation (SpO2) is the amount of oxyhemoglobin expressed as a percentage
of the hemoglobin that is available to transport oxygen.
Pulse Rate (PR)
Pulse rate (PR), measured in beatsperminute (BPM), is based on the optical detection of peripheral
flow pulse.
Perfusion Index (PI)
The Perfusion Index (PI) is the ratio of the pulsatile blood flow to the non-pulsatile or static blood
in peripheral tissue. PI thus represents a noninvasive measure of peripheral perfusion that can be
continuously and noninvasively obtained from a pulse oximeter.
Plethysmograph
Waveform (Pleth)
The amount of arterial blood in tissue changes with your pulse (photoplethysmography). Therefore,
the amount of light absorbed by the varying quantities of arterial blood changes as well. Clinicians
have attempted to utilize this waveform and its variation for assigning signal integrity,
physiological and artifactual changes such as perfusion changes, dysrhythmia, motion artifact, and
electrical interference. For this reason, accurate and reliable presentation of the plethysmograph
waveform is of importance.
Bar graph
Showing the instantaneous state of blood pulse which is proportional to pulse intensity.
2.1.2 Intended Use
The AI-HRV is indicated for the noninvasive spot checking of blood flow and functional oxygen
saturation of arterial hemoglobin (SpO2) and pulse rate (PR) for adult and pediatric (≥10Kg) patients
during both no motion and motion conditions, and for patients who are well or poorly perfused.
2.1.3 Population
Adult and pediatric (≥10Kg).
2.1.4 Indication for Environment
AI-HRV is not intended for hospitals, hospital-type facilities, is only intended for home environments.

BioSignals LIMITED. All Rights Reserved. Page 8 of 19
2.1.5 Operator
Individuals (Consult clinical professionals before using).
2.1.6 Model of Operation
Spot-check.
2.1.7 Measurement Position
Finger.
2.1.8 Indications
None.
2.1.9 Contra-indications
None.
2.1.10Side-effects
None.
2.2 Product Display
2.2.1 AI-HRV Display
3. Unit of pulse rate: PR/min
4. Measured value of pulse rate: the display range is [25 ~ 250] and the sign “---” means invalid value.
5. Unit of perfusion index: PI%
6. Measured value of perfusion index: the display range is [0.02 ~ 20.0]and the sign “---” means invalid
value.
7. Bar chart: showing the instantaneous state of blood pulse which is proportional to pulse intensity.
8. Tracing wave: display physiological pulse waveform, and non-normalized processing.
9. Battery capacity: the indicator’s lighting length varies with remaining battery capacity.
7
8
9
3
5
6
4

BioSignals LIMITED. All Rights Reserved. Page 9 of 19
3 Basic Operation
3.1 Installment
3.1.1 Battery Installment
1. Ensure the polarity of two AAA batteries in consistent with the polarity mark in the battery compartment.
2. Place two AAA batteries into the battery compartment gently.
3. Make the battery compartment covered rightly.
Note: ensure correct polarity when install batteries.
3.2 Navigating the Menu
From the Main Screen, press the button to access the Menu.
Display Button
Menu Options
Description
Manual Direction Rotation
Allows the user to switch screen display direction.
Parameter Trend
Allows the user to review parameter trend during measurement period.
Settings
Enter the menu.
Power Off
Power off the pulse oximeter.
Exit
Exit the selected screen.
Sensitivity Settings
Sensitivity under low perfusion condition, default is ‘MID’.
Average Settings
Parameter average times, default is 6 seconds.
Automatic Direction Rotation
Enable/Disable automatic screen display direction.
Brightness Settings
Screen brightness, default is ‘MID’.
Saturation Trend
SpO2trend during measurements.
Pulse Rate Trend
Pulse rate trend during measurements.
BioSignals LIMITED. All Rights Reserved. Page 10 of 19
4 Measurement
4.1 Alarm
1. Carefully check the measurement part before wearing the AI-HRV. Do not wear the AI-HRV to the
damaged skins or tissues.
2. Do not place the AI-HRV on the side of body with artery catheter or intravenous injection tube.
3. Do not put the AI-HRV and blood pressure cuff on the same side of body as the blood flow occlusion
during blood pressure measurement may affect the reading of blood volume pulse.
4.2 Measurement Procedure
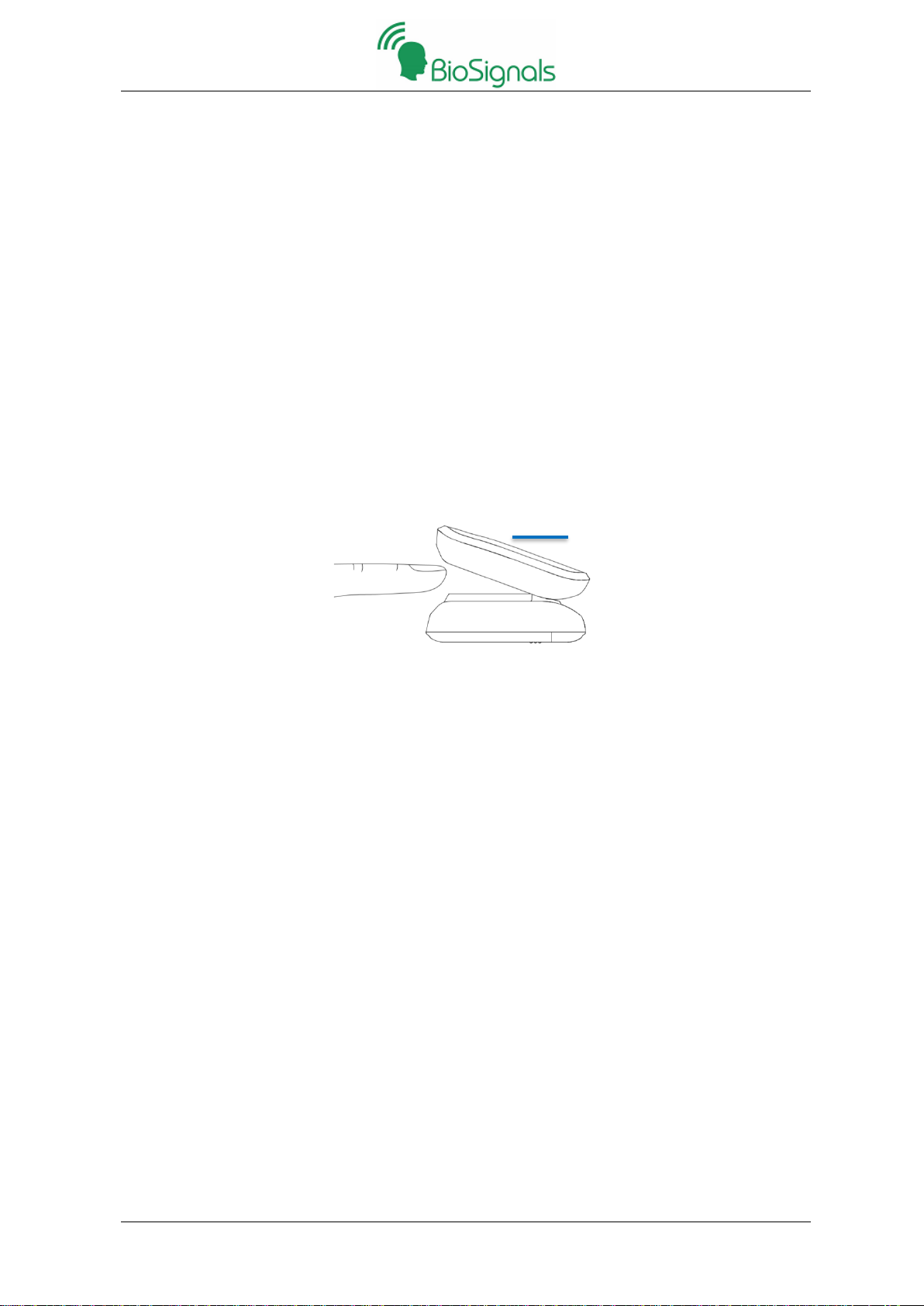
1. Press ON/OFF Button to turn on the AI – HRV.
2. Open the finger clip, put the finger into cavity, and then release the finger clip. Make the display screen
above the finger clip when putting the finger.
As shown in the figure below:
4.3 Measurement Precautions
1. Put the proper finger into the AI-HRV. The measured value may be affected if the finger is too cold or
thin.
2. The finger shall be inserted to the finger clip cavity deeply enough, and otherwise, the measured value
may be inaccurate. Ensure the light between the photoelectric receiving tube and the luminescent tube of
the AI-HRV shall passes through the small artery bed of the finger of the subject.
3. Keep the place where the light path passes free from light barriers such as adhesive tape to ensure the
accuracy of parameter measurement.
4. The product is suitable for children and adults with finger thickness of 7mm ~ 25.4mm.
5. The recommended measurement position are: forefinger, middle finger, ring finger.
6. The measurement will be affected by excessively strong ambient light, including but not limited to
fluorescent lamp, double Ruby lamp, infrared heater, direct sunlight, etc.
7. The measurement accuracy will be affected if the subject performs strenuous activities.
8. The interference of electrical surgical equipment may affect the measurement accuracy.
9. The nail on the test site shall be free from nail polish and other cosmetics. To ensure measurement
accuracy, remove nail polish or other cosmetics if any.
10. The measured value of blood volume pulse may be inaccurate in case there is high content of the dye
dilution drugs (such as methylene blue, indigo cyanide green, acid indigo carmine), carbon monoxide
hemoglobin, methemoglobin or Thoi hemoglobin and others in the body of the subject.
Screen

BioSignals LIMITED. All Rights Reserved. Page 11 of 19
11. Dopamine, Procaine, Procaine, Lidocaine, Benzocaine, and other drugs will cause serious deviation in
the measurement of blood flow and blood oxygen saturation.
12. The measured value of blood oxygen saturation is for reference only to anemic hypoxia and toxic
hypoxia. Some patients with severe anemia may still have higher measured value of blood oxygen
saturation due to physiological reasons.
13. SpO2 accuracy is not assured in those with arrythmias.

BioSignals LIMITED. All Rights Reserved. Page 12 of 19
5 Maintenance
Regular maintenance is of great importance to ensure the normal operation of equipment.
Warning
1. The integration and verification of software function have been completed before the product leaving
the factory, and the user does not need to install, upgrade, or maintain.
2. All safety inspection or maintenance work of equipment to be disassembled shall be carried out by
professional maintenance personnel, and the non-professional personnel may cause equipment failure.
3. If there is any problem with the equipment, please contact the maintenance personnel or our company.
5.1 Cleaning and Disinfection
Keep the equipment clean and observe the following regulations to prevent equipment from being
damaged:
1. Use the cleaning disinfectant designated by our company to clean and disinfect the equipment according
to the method regulated in the manual, and otherwise the product performance or service life may be
affected.
2. Forbid to immerse the equipment in liquid.
3. Forbid to dump liquid on the equipment.
4. Do not make liquid into to enter frame of equipment.
5. Forbid to use abrasive materials (such as steel velvet or silver polishing agent), and any strong solvent
(such as acetone or detergent containing acetone component).
6. High temperature and high pressure or gas disinfection method are not inapplicable to disinfect the
equipment.
Warning
7. The equipment shall be cleaned or disinfected with materials and methods listed in this chapter. Our
company shall not assume any responsibility for damage caused by the using other materials or methods.
8. Our company shall not assume any responsibility for the effectiveness of the listed chemicals or methods
as a means of infection control. If used in a hospital, consult the infection prevention department or
epidemiologist of the hospital.
5.1.1 Cleaning
The equipment shall be cleaned regularly. In case the equipment is used in areas with serious
environmental pollution or windy sand, the equipment shall be cleaned more frequently.
The following cleaning agents are available:
➢70% ethanol
➢Clean water
When cleaning the equipment:
1. Shut down the equipment and take out batteries.
2. Make a soft cotton ball absorb a proper amount of detergent, and then use it to wipe the display
screen.
BioSignals LIMITED. All Rights Reserved. Page 13 of 19
3. Make a soft cloth absorb a proper amount of detergent, and then use it to wipe the surface of the
equipment.
4. If necessary, use a dry cloth to wipe off excess detergent.
5. Make the equipment dry in a cool and ventilated environment.
5.1.2 Disinfection
To disinfect the equipment may cause certain damage to the appearance of AI-HRV. It is recommended
to disinfect the equipment only when necessary.
The recommended disinfectants include 70% ethanol.
5.2 Maintenance
The design life of the product is 5 years. Please maintain the equipment as follows:
1. Clean and disinfect the equipment according to this instruction before using.
2. Replace batteries in time when the low battery indicator appears.
3. Take out batteries if the equipment will not be used for a long time.
4. The equipment shall be stored indoor, with temperature at - 20℃~ + 60 ℃, relative humidity no
higher than 95%, free of corrosive gas and good ventilation. Moisture and light may affect the service
life of the equipment, and even induce equipment damaged.
5.3 Troubleshooting
Only professional maintenance personnel are allowed to disassemble the AI-HRV. There are no parts inside
the equipment to be adjusted.
Malfunction
Possible Cause
Solution
Unable to turn on the
equipment
Low or dead battery
Replace batteries
No install batteries correctly
Install batteries correctly and ensure correct
polarity
Equipment is damaged
Contact the local customer service center
Pulse rates fail to display
Not insert fingers correctly
Put fingers correctly
The user’s fingers are too thin, resulting in
light leakage.
Put a thicker finger
The user’s finger is cold so that the blood
perfusion is too low.
Keep the finger warm and put another
finger for measurement.
nail polish or manicure
Remove nail polish or manicure
Unstable display of pulse
rate
No insert finger deep enough
Put the finger correctly
Movement interfaces signal
Stay still
LED goes out suddenly
The product is designed to shut down
automatically when no signs is detected.
Normal
Low battery
Replace battery

BioSignals LIMITED. All Rights Reserved. Page 14 of 19
6 Specification
6.1 Product Specification
1. The product uses internal power supply.
2. Power requirements: 2 AAA batteries.
3. Liquid inlet protection degree: IP22.
4. Operation mode: run continuously.
5. Electrical safety classification: BF type application part.
6. Not suitable to use in places with flammable anesthetic gas.
7. Normal working conditions: ambient temperature range: 0 ~ 40 ℃; ambient humidity range: 15% ~ 95%
non-condensing; atmospheric pressure range: 79.5kPa ~ 107.4kpa.
8. Storage environment: environment temperature range: -20 ~ 60 ℃; environment humidity range: 15%
~ 95%, non-condensing; atmospheric pressure range: 79.5kPa ~ 107.4kpa.
9. Physical dimension: 62mm × 35mm × 31mm.
10. Product weight: ≤75g (including battery).
11. Parameters of LED light source.
Light Source
Central Wavelength
Radiant Power
Red light
660nm
<15mW
Infrared light
905nm
<15mW
6.2 Parameter Specification
Functional Oxygen Saturation (SpO2):
Condition
Range
ARMS*
No Motion[1]
70% - 100%
2%
Motion[2]
70% - 100%
3%
Low Perfusion[3]
70% - 100%
2%
Range [0% - 69%] is undefined.
Pulse Rate (PR):
Condition
Range
ARMS*
No Motion[4]
25 bpm - 250 bpm
2 bpm
Motion[4]
25 bpm - 250 bpm
4 bpm
Low Perfusion[4]
25 bpm - 250 bpm
2 bpm
*Arms accuracy is statistical calculation of the difference between device measurements and reference measurements.
Approximately two-thirds of the device measurements fell within +/– Arms of the reference measurements in controlled
study.
1. The AI-HRV has been validated for no motion accuracy in human blood studies on healthy adult male and female
volunteers with light to dark pigmented skin in induced hypoxia studies in the range of 70% - 100% against a laboratory
co-oximeter.
2. The AI –HRV has been validated for motion accuracy in human blood studies on healthy adult male and female
volunteers with light to dark pigmented skin in induced hypoxia studies while performing rubbing and tapping motions, at

BioSignals LIMITED. All Rights Reserved. Page 15 of 19
2 ~ 4 Hz at an amplitude of 1 ~ 2 cm and a non-repetitive motion between 1 ~ 4 Hz at an amplitude of 1 ~ 2 cm in the range
of 70% - 100% against a laboratory co-oximeter.
3. The AI-HRV has been validated for low perfusion accuracy in bench top testing against a Fluke Index II simulator with
signal strengths of greater than 0.025% and transmission of default level 4000 for saturations ranging from 70% ~ 100%.
4. The AI –HRV has been validated for pulse rate accuracy for the range of 25 ~ 250 bpm in bench top testing against a
Fluke Index II simulators with signal strengths of greater than 0.025%and transmission of default level 4000 for saturations
ranging from 70% ~ 100%. Pulse rate accuracy under motion was verified by bench top testing in the range of 45 ~ 180
bpm against a Fluke Index II simulator using the motion preset settings (Preset Motion Type: Leve_0 and Level_1).
5. Plethysmogram
Provide non-normalized plethysmogram.
6. Bargraph
Bargraph is an instantaneously physiological index, which the maximum amplitude reflects the strength
of blood perfusion and the rhythm reflect the stability of the physiological signal.
7. Update period
The data update period is less than 16s. The default average time is 6s, and provide 2s, 4s, 6s, 8s, 10s, and
12s levels for user selection. Saturation and pulse rate are routinely calculated for each second.
Short average time selection will get good physiological following feature, while long average time
selection will get steadily physiological output by restrict the short-term physiological fluctuation.
8. Signal incompleteness
There will prompt invalid output as “--” when parameters turned into invalid state during bad condition
such as too large noise signal, bad signal quality, or physiological signal disappear.
9. Signal Sensitivity
Signal Sensitivity allows users to select the probability of valid parameter under low perfusion condition.
High sensitivity means more parameter output under low perfusion condition, which might increase
probability of unreliable parameter value. Low sensitivity means less parameter output under low
perfusion condition, which can reduce probability of unreliable parameter value. Use high sensitivity
settings will get fast respond to the parameter, otherwise low sensitivity will get slow respond to the
parameter.
According to industry convention, low perfusion condition is defined as less than 0.3%. Default sensitivity
configuration is recommended for most population. Anyway, if people in week perfusion condition
(<0.3%), high sensitivity might be useful.

BioSignals LIMITED. All Rights Reserved. Page 16 of 19
7 Performance
7.1 Summary
According to the principle of ISO80601-2-61 and AI-HRV were selected as representive for performance
trial investigation, and both quiet state and motion state are studied in this trial. The motion types are
defined as follows:
Type I: Rubbing & Tapping, Frequency [2 ~ 4] Hz, amplitude [1 ~ 2] cm.
Type II: Non-Repetitive Motion, Frequency [1 ~ 4] Hz, amplitude [1 ~ 2] cm.
Totally 13 subjects were enrolled in this clinical trial. Among them, 3 cases were male, 10 cases were
female, 2 cases were dark-skinned, 3 cases were light-skinned, and aged from 21 to 33 years old.
7.2 Results
Estimate the accuracy based on the root mean square (Arms) of measurements obtained by the AI-HRV and
the reference equipment. The results are as follows:
Pulse Oximeter
Accuracy (Arms) with Range (%)
[100-60]
[100-90]
(90-80]
(80-70]
(70-60]
AI – HRV (Quiescence)
1.61
1.20
1.70
1.87
2.31
AI -HRV (Motion)
1.53
1.25
1.60
1.56
2.41
7.3 Conclusion
The study was conducted as expected with no adverse events and no deviations from the protocol.
The measurement parameters of the AI -HRV meets the requirement of the standard ISO 80601-2-61 and
YY0784during both quiet state and motion state. The SpO2 accuracy of AI – HRV is equivalent to the
invasive blood gas measurement results, and the pulse rate accuracy is equivalent to the heart rate
measurement from an ECG monitor.

BioSignals LIMITED. All Rights Reserved. Page 17 of 19
8 EMC Specification
8.1 Instructions for Use
The ME EQUIPMENT or ME SYSTEM is suitable for home healthcare environments and so on.
Warning: Don’t near active HF surgical equipment and the RF shielded room of an ME system for
magnetic resonance imaging, where the intensity of EM disturbances is high.
Warning: Use of this equipment adjacent to or stacked with other equipment should be avoided because
it could result in improper operation. If such use is necessary, this equipment and the other equipment
should be observed to verify that they are operating normally.
Warning: Use of accessories, transducers and cables other than those specified or provided by the
manufacturer of this equipment could result in increased electromagnetic emissions or decreased
electromagnetic immunity of this equipment and result in improper operation.”
Warning: Portable RF communications equipment (including peripherals such as antenna cables and
external antennas) should be used no closer than 30 cm (12 inches) to any part of the AI-HRV including
cables specified by the manufacturer. Otherwise, degradation of the performance of this equipment could
result.
If any: a list of all cables and maximum lengths of cables (if applicable), transducers and other
ACCESSORIES that are replaceable by the RESPONSIBLE ORGANIZATION and that are likely to
affect compliance of the ME EQUIPMENT or ME SYSTEM with the requirements of Clause 7
(EMISSIONS) and Clause 8 (IMMUNITY). ACCESSORIES may be specified either generically (e.g.,
shielded cable, load impedance) or specifically (e.g., by MANUFACTURER and EQUIPMENT OR TYPE
REFERENCE).
If any: the performance of the ME EQUIPMENT or ME SYSTEM that was determined to be ESSENTIAL
PERFORMANCE and a description of what the OPERATOR can expect if the ESSENTIAL
PERFORMANCE is lost or degraded due to EM DISTURBANCES (the defined term “ESSENTIAL
PERFORMANCE” need not be used).
8.2 Technical Description
1.All necessary instructions for maintaining BASIC SAFETY and ESSENTIAL PERFORMANCE with
regard to electromagnetic disturbances for the excepted service life.
2. Guidance and manufacturer’s declaration -electromagnetic emissions and Immunity.
Guidance and Manufacturer’s Declaration - Electromagnetic Emissions
Emissions test
Compliance
RF emissions (CISPR 11)
Group 1
RF emissions (CISPR 11)
Class B
Harmonic emissions (IEC 61000-3-2)
Not application
Voltage fluctuations/ flicker emissions (IEC 61000-3-3)
Not application

BioSignals LIMITED. All Rights Reserved. Page 18 of 19
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity (1)
Immunity Test
IEC 60601-1-2 Test level
Compliance level
Electrostatic discharge (ESD)
IEC 61000-4-2
±8 kV contact
±2 kV, ±4 kV, ±8 kV, ±15 kV air
±8 kV contact
±2 kV, ±4 kV, ±8 kV, ±15 kV air
Electrical fast transient/burst
IEC 61000-4-4
Power supply lines: ±2 kV
input/output lines: ±1 kV
100 kHz repetition frequency
Not application
Surge
IEC 61000-4-5
line(s) to line(s): ±1 kV.
line(s) to earth: ±2 kV.
Not application
Voltage dips, short interruptions and
voltage variations on power supply input
lines
IEC 61000-4-11
0% 0.5 cycle
At 0º, 45 º, 90 º, 135 º, 180 º, 225 º, 270 º
and 315 º
0% 1 cycle
And
70% 25/30 cycles
Single phase: at 0
0% 300 cycles
Not application
Power frequency magnetic field
IEC 61000-4-8
30 A/m
50Hz/60Hz
30 A/m
50Hz/60Hz
Conduced RF
IEC61000-4-6
150KHz to 80MHz:
3Vrms
6Vrms (in ISM and amateur radio bands)
80% Am at 1kHz
Not application
Radiated RF
IEC61000-4-3
10 V/m
80 MHz – 2,7 GHz
80 % AM at 1 kHz
10 V/m
80 MHz – 2,7 GHz
80 % AM at 1 kHz
NOTE UT is the a.c. mains voltage prior to application of the test level.
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity (2)
Radiated RF
IEC61000-4-3
(Test specifications
for ENCLOSURE
PORT IMMUNITY to
RF wireless
communications
equipment)
Test
Frequency
(MHz)
Band
(MHz)
Service
Modulation
Modulation
(W)
Distance
(m)
IMMUNITY
TEST LEVEL
(V/m)
385
380 –
390
TETRA 400
Pulse
modulation
18 Hz
1,8
0.3
27
450
430 –
470
GMRS 460,
FRS 460
FM
± 5 kHz
deviation
1 kHz sine
2
0.3
28
710
704 –
787
LTE Band 13,
17
Pulse
modulation
217 Hz
0,2
0.3
9
745
780

BioSignals LIMITED. All Rights Reserved. Page 19 of 19
810
800 –
960
GSM
800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
Pulse
modulation
18 Hz
2
0.3
28
870
930
1720
1 700 –
1 990
GSM 1800;
CDMA 1900;
GSM 1900;
DECT;
LTE Band 1, 3,
4, 25; UMTS
Pulse
modulation
217 Hz
2
0.3
28
1845
1970
2450
2 400 –
2 570
Bluetooth,
WLAN,
802.11 b/g/n,
RFID 2450,
LTE Band 7
Pulse
modulation
217 Hz
2
0.3
28
5240
5 100 –
5 800
WLAN 802.11
a/n
Pulse
modulation
217 Hz
0,2
0.3
9
5500
5785
Table of contents