2
Boston Scientific (Master Brand DFUTemplate 3in x 9in Global, 91031819AA), DFU, MB, Solyx, Global, 50431073-01A
Black (K) ∆E ≤5.0Black (K) ∆E ≤5.0
Table of ConTenTs
WaRnInG ...........................................................................................3
DeVICe DesCRIPTIon.......................................................................3
InTenDeD Use/InDICaTIons foR Use .......................................3
ConTRaInDICaTIons...................................................................... 3
GeneRal WaRnInG.........................................................................4
PosT PRoCeDURal WaRnInG...................................................... 4
PReCaUTIons....................................................................................4
aDVeRse eVenTs .............................................................................5
HoW sUPPlIeD..................................................................................5
oPeRaTIonal InsTRUCTIons.......................................................5
Prior to Use....................................................................................5
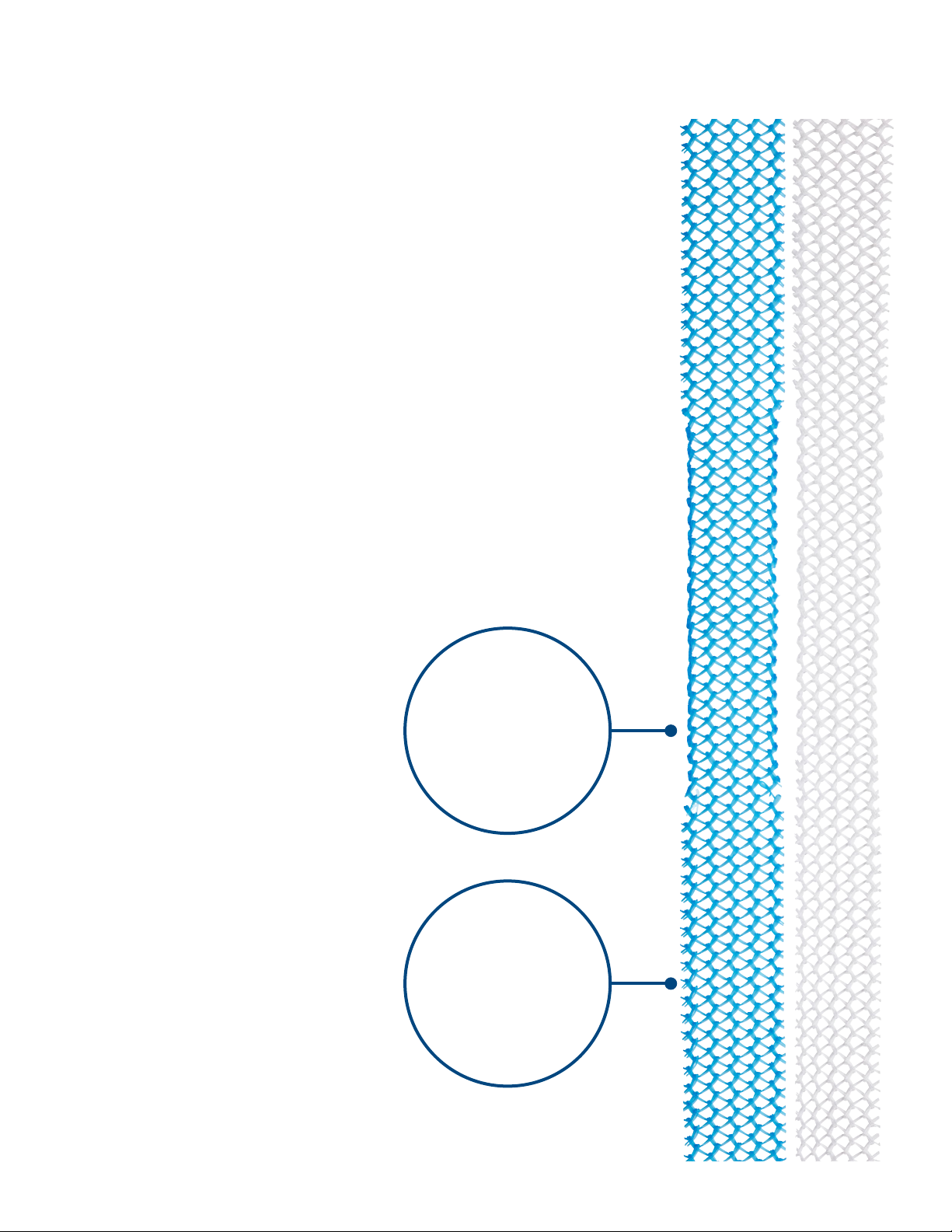
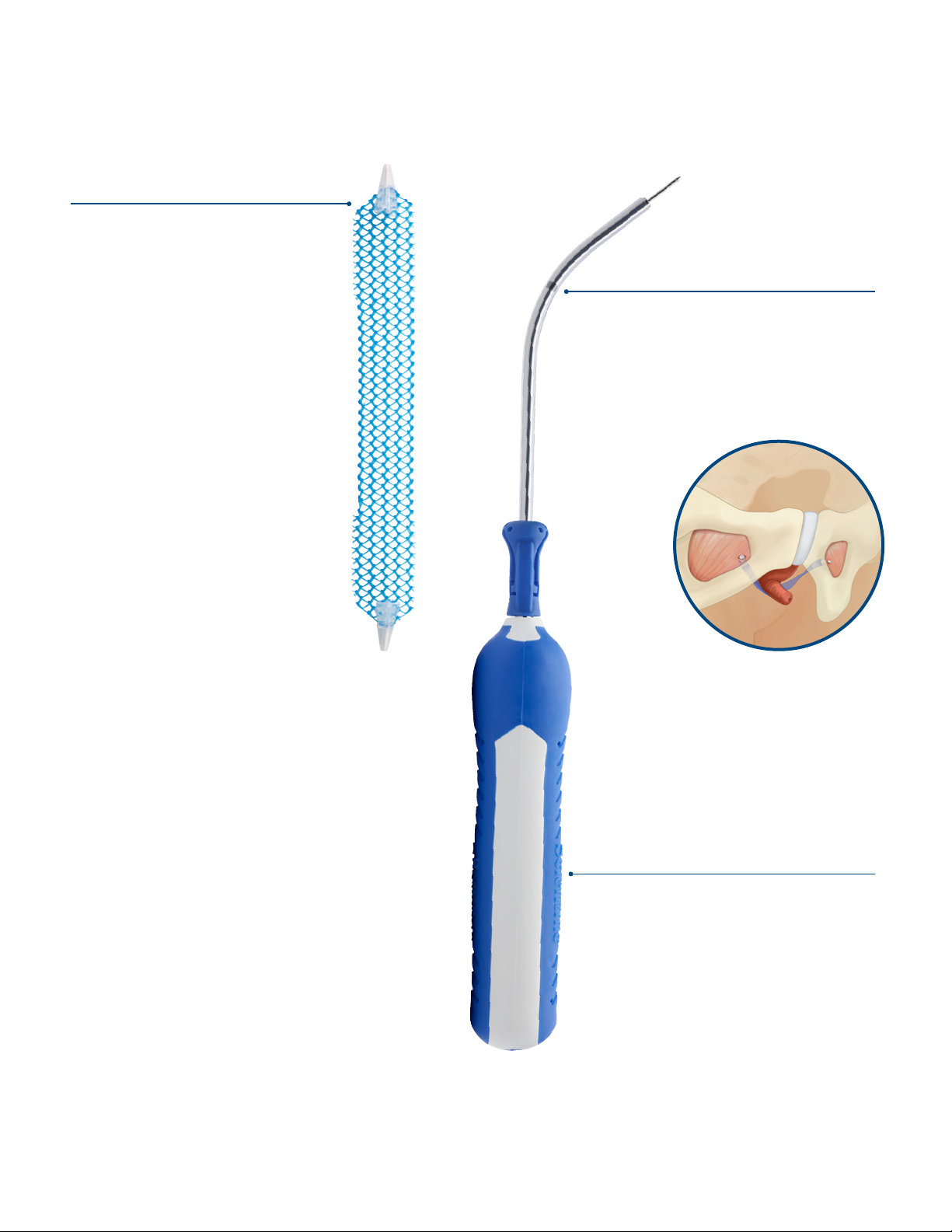
Figure 1: Solyx™ SIS System and Solyx Blue SIS
System...................................................................................... 6
WaRnInG ..........................................................................................6
Steps for Use.................................................................................6
Incision and Dissection............................................................... 6
Figure 2: Dissection Pathway...............................................6
Sling Placement ...........................................................................6
PReCaUTIon...................................................................................... 7
Figure 3: Mesh Assembly Placement onto Delivery
Device ......................................................................................7
Figure 4: Mesh Orientation on Delivery Device.................7
WaRnInG ...........................................................................................7
Figure 5..................................................................................... 8
Figure 6..................................................................................... 8
Figure 7..................................................................................... 8
Figure 8: Contralateral Delivery Device Placement..........9
WaRRanTY ........................................................................................9