diapath Donatello User manual

Donatello
TM
Tissue Processor
User Manual

2
Manufacturer:
Diapath S.p.A.
Via Savoldini,71
24057 Martinengo (BG) Italy
Tel. (+39)0363.986.411
Fax (+39)0363.948.000
www.diapath.com
info@diapath.com

3
Manual information
This document is protected by copyright laws; copyrights of this document belong to Diapath S.p.A.
It is strictly forbidden, full or in part, to modify this manual in absence of an explicit authorization.
Diapath S.p.A. is not obliged to regulate updates of this manual, or to send regularly reviewed or updated versions of
this manual to its customers.
Information contained in this manual is subject to change without prior notice.
Diapath S.p.A. guarantees the accuracy of the information present in this manual as a result of thorough research; if
information is incorrect or unclear, please contact Diapath S.p.A. or the distributor. Diapath S.p.A. is not responsible for
any kind of material damages or other damages related to non-compliance with indications or specific information
present in this manual.
Diapath S.p.A. continuously adopts a development policy and reserves the right to make changes and improvements to
any product described in this manual without prior notice.
The serial number and date of manufacture are reported on the registration plate attached on the rear part of the
instrument.
Inspired to:
Donatello, Donato di Niccolò di Betto Bardi
Donatello (Florence, 1386 – Florence, 13 Dec. 1466), was an Italian sculptor, goldsmith and
designer. With his long career was one of the three fathers of the Florentine Renaissance, along
with Filippo Brunelleschi and Masaccio, and one of the most celebrated sculptors of all time.
Gave a fundamental contribution to the renewal of the ways of the sculpture, setting aside the
experiences of the late Gothic and overcoming the classic models of Roman art, the sign of a
new and restless expressionism that pervades his best works.
David, 1440 –
Most famous Donatello sculpture
Bargello Museum - Florence
Manual revision: 5
Edition date: July 30
th
2018

4
Contents
Manual information.......................................................................................................................................................................... 3
Contents ......................................................................................................................................................................................... 4
Preface ........................................................................................................................................................................................... 6
Destination of use ........................................................................................................................................................................... 6
Quality control................................................................................................................................................................................. 7
Unpacking and operation ............................................................................................................................................................... 12
Instrument placement and installation ............................................................................................................................................ 12
1 Donatello
TM
Tissue Processor components ..................................................................................................................... 19
1.1 Instrument overview ............................................................................................................................................................ 19
1.2 Instrument rear part ............................................................................................................................................................... 20
1.3 Instrument features ................................................................................................................................................................. 20
1.4 Parts supplied – Packing list ..................................................................................................................................................... 22
1.5 Technical data ....................................................................................................................................................................... 23
1.6 Processing chamber (SPC) .................................................................................................................................................... 24
1.6.1 Processing capacity ..................................................................................................................................................... 24
1.6.2 Level sensors .............................................................................................................................................................. 24
1.6.3 Half load mode ............................................................................................................................................................ 25
1.7 Wax reservoir ........................................................................................................................................................................ 25
1.7.1 Cleaning wax system ................................................................................................................................................... 25
1.7.2 Exhausting wax dumping system (EWD/PWD) ............................................................................................................... 26
1.8 Reagent stations................................................................................................................................................................... 26
1.8.1 Compatible reagents ................................................................................................................................................... 27
1.8.2 Reagent management module ................................................................................................................................... 27
1.8.3 DAF (Decreasing Aging Factor) .................................................................................................................................... 28
1.8.4 Remote reagent fill and drain (RFill/RDrain) ............................................................................................................... 29
1.9 Fast Processing system (FPS) ................................................................................................................................................... 29
2 DonatelloTM tissue processor software operation ............................................................................................................. 30
2.1 Main screen.......................................................................................................................................................................... 30
2.1.1 Main screen upper area ............................................................................................................................................... 30
2.1.2 Main screen central area ............................................................................................................................................ 31
2.1.3 Main screen lower area .............................................................................................................................................. 35
2.2 Instrument status .................................................................................................................................................................. 36
2.3 Warnings and alarms ............................................................................................................................................................. 36
2.4 Tank position status.............................................................................................................................................................. 37
2.5 Starting processing ................................................................................................................................................................ 38
2.5.1 Normal processing ...................................................................................................................................................... 38
2.5.2 Safety procedures warning messages............................................................................................................................. 39
2.5.3 Inverted processing ...................................................................................................................................................... 39
2.5.4 Cassette tracking .......................................................................................................................................................... 39
2.5.5 Choice of processing end time ....................................................................................................................................... 40
2.5.6 Starting processing ....................................................................................................................................................... 41
2.5.7 Bubbling ...................................................................................................................................................................... 42
2.6 Washing ................................................................................................................................................................................ 43
2.6.1 Starting washing ........................................................................................................................................................... 43
2.7 Reagent replacement ............................................................................................................................................................. 44
2.7.1 Tank replacement ......................................................................................................................................................... 45
2.7.2 New tank ..................................................................................................................................................................... 49
2.7.3 Filter support system .................................................................................................................................................... 51
2.8 Main menu ............................................................................................................................................................................ 52
2.8.1 Instrument information ................................................................................................................................................. 53
2.8.2 General information ...................................................................................................................................................... 53
2.8.3 Diapath Setting ............................................................................................................................................................ 54
2.8.4 Modification of processing and washing procedures ........................................................................................................ 56
2.8.5 Manual operation .......................................................................................................................................................... 58
2.8.6 Reagent management ................................................................................................................................................... 61
2.8.7 MultiSense ................................................................................................................................................................. 64
2.8.8 Statistics menu ............................................................................................................................................................. 65
2.8.9 User management ........................................................................................................................................................ 70
3 Specimens Traceability .................................................................................................................................................... 74
3.1 Overview ................................................................................................................................................................................. 74
3.2 Specimen insertion ................................................................................................................................................................ 74
3.3 Statistics ................................................................................................................................................................................. 76

5
4 Safety procedures.................................................................................................................................................................. 77
4.1 Safe procedure – Safe reagent ................................................................................................................................................. 77
4.2 Restart .................................................................................................................................................................................. 81
5 Alarms and warnings.............................................................................................................................................................. 84
5.1 Not blocking alarms .............................................................................................................................................................. 84
5.2 Blocking alarms .................................................................................................................................................................... 87
6 Cleaning and maintenance ................................................................................................................................................... 90
6.1 Maintenance Schedule ............................................................................................................................................................. 90
6.2 Instrument decontamination procedure ..................................................................................................................................... 91
6.2.1 Preventative measures and IPD to use ............................................................................................................................. 91
6.2.2 General information on sanitizing solution ........................................................................................................................ 91
6.2.3 Decontamination procedure............................................................................................................................................. 91
6.3 LCD display cleaning ................................................................................................................................................................ 92
6.4 External surfaces and process chamber cleaning........................................................................................................................ 92
6.5 Charcoal filter replacement ....................................................................................................................................................... 92
6.6 Lid gasket maintenance............................................................................................................................................................ 94
6.7 Fuses replacement ................................................................................................................................................................... 94
6.8 Preventive maintenance ........................................................................................................................................................... 95
7 Accessories ............................................................................................................................................................................ 96
8 Troubleshooting..................................................................................................................................................................... 97
9 WEEE Directive ...................................................................................................................................................................... 98
9.1 Uninstallation and disposal ....................................................................................................................................................... 98
10 Diapath instrument warranty ............................................................................................................................................. 98

6
Preface
Destination of use
Donatello
TM
is an automatic smart tissue processor for lab use.
Its use is intended for fixation, dehydration and wax infiltration of histological specimen tissues.
The instrument can be used, accordingly to all instruction on this manual, only by well-trained and authorized staff.
Any improper use of the instrument must be considered as an operation that is not allowed.
Maintaining and reading of this manual is necessary for the use of the instrument in order to avoid dangerous situations.
The operators shall be adequately trained in its use and must respect all safety rules and its use limits.
Trained and authorized Diapath S.p.A. staff shall perform instrument maintenance, due to trouble or malfunction.
The instrument complies with CISPR 11 Class A rules: in case of domestic use, it may cause radio interferences. In this case, a
measurement and mitigation of such interference is necessary.
Before installing the instrument, an analysis of electromagnetic interferences is performed.
Do not use the device near sources of strong electromagnetic radiations, such as radiofrequency waves generators. In this case the
radiations may interfere with normal functioning of the instrument.
Here below all regulations followed for the development and manufacturing of Donatello
TM
Tissue Processor:
·Directives 98/79/CE of European Parliament on medical devices and medical devices in use
·Directive 2014/53/EU of the European Parliament and of the council of 16 April 2014 on radio equipment
·Legislative Decree 27th January 2010, n. 17 implementing Directive 2006/42/EC on machinery and amending Directive
·95/16/EC relating to lifts. ISO9001(*) Quality management system requirements
·ISO 13485(*): Quality management system requirements for medical devices. Requirements for regulatory purposes and
specific requirements of the application of ISO 9001(*)
·UNI CEI EN ISO 14971(*): application of risks management to medical devices
·EN ISO 18113-3(*): In vitro diagnostic medical devices. Information supplied by the manufacturer (labelling). Part 3: In vitro
diagnostic instruments for professional use.
·EN ISO 15223-1(*): Symbols to be used with medical device labels, labelling and information to be supplied. Part 1: General
requirements.
·EN 61326-1(*): Electrical equipment for measurement, control and laboratory use - EMC requirements
Part 1: General requirements.
·EN 61326-2-6(*): Electrical equipment for measurement, control and laboratory use - EMC requirements. Part 2-6: Particular
Requirements - In vitro diagnostic medical devices (IVD).
·EN 61000-3-2(*): Electromagnetic compatibility (EMC) Part 3-2: Limits - Limits for harmonic current emissions. (Equipment
with input current <= 16A per phase).
·EN 61000-3-3(*)+ A1(*)+ A2(*): Electromagnetic compatibility (EMC) Part 3-3: Limits - Limitation of voltage fluctuations and
of flicker in low voltage supply systems for equipment with rated current <= 16A and not subject to conditional connection.
·EN 61010-1(*): Safety requirements for electrical equipment for measurement, control and laboratory use. Part 1: General
requirements.
·EN 61010-2-101(*): Safety requirements for electrical equipment for measurements, control and laboratory use. Part 2-101:
Particular requirements for medical equipment for in-vitro diagnostics.
·EN 62471(*) LED light – Category risk group 1.
·ETSI EN 301 489-1(*) Part 1: General requirements; Harmonized standard covering the essential requirements of article 3.1 (b)
of Directive 2014/53/EU and essential requirements covering the essential requirements of article 6 of directive 2014/30/EU
·ETSI EN 301 489-3(*) Part 3: Electromagnetic Compatibility (EMC) standard for radio equipment and services; Part 3: Specific
conditions for Short-Range Devices (SRD) operating on frequencies between 9 kHz and 246 GHz; Harmonized standard covering
the essential requirements of article 3.1(b) of Directive 2014/53/EU
· ETSI EN 300 330-1(*): Electromagnetic compatibility and Radio spectrum Matters (ERM); Short Range Devices (SRD); Radio
equipment in the frequency range 9 kHz to 25 MHz and inductive loop systems in the frequency range 9 kHz to 30 MHz; Part 1:
Technical characteristics and test methods
·ETSI EN 300 330-2(*): Electromagnetic compatibility and Radio spectrum Matters (ERM); Short Range Devices (SRD); Radio
equipment in the frequency range 9 kHz to 25 MHz and inductive loop systems in the frequency range 9 kHz to 30 MHz; Part 2:
Harmonized EN covering the essential requirements of article 3.2 of the R&TTE Directive.
·EN 50364(*)Limitation of human exposure to electromagnetic fields from devices operating in the frequency range 0 Hz to 300
GHz, used in Electronic Article Surveillance (EAS), Radio Frequency Identification (RFID) and similar applications
(*)
In the applicable edition

7
Quality control
Diapath S.p.A. adopts quality management system ISO 9001(*) and ISO 13485(*) to design, make and improve the technical-scientific
solution for the customer in medical and diagnostic in vitro systems, assuring high quality services with the help of an highly qualified
and motivated staff to contribute to the improvement of Diapath S.p.A. products.
Diapath S.p.A. constantly looks for customer’s satisfaction, tries to perceive their expectations and to treat the customer without any
discrimination. Diapath S.p.A. assures the application of normative requirements.
Quality assurance of data furnished by Diapath S.p.A. is obtained through these activities:
·Use only reference material
·Execution of proofs of the whole production
·Correlation of different results
The internal quality control foresees to monitor the most difficult passages of an analytic process so as to verify their stability, assuring
that quality parameters estimated during the validation or check of the method are valid also for real instruments and that they will not
worsen during the course of the time.
Diapath S.p.A. tests its own products according to some internal procedures, to reduce possibility of anomalies during the operation.
To identify and to find out its own instruments, Diapath S.p.A. uses some internal procedures to find production recording, assembling
recording, tests recording, serial numbers or batch codes and configuration recording.
Instrument information
All information present in this manual is only regarding the device shown on the cover.
On instrument, there is the registration plate with the serial number (Picture 1).
Storage and handling
For a correct storage and function of the instrument, all instructions provided in this manual concerning maintenance and installation
should be respected.
Also the showed below environmental requirements should be respected:
The instrument can work with 80% relative humidity conditions for temperatures up to 31 °C with a linear decrease up to 50% at the
temperature of 40° C. Voltage variation can’t exceed +/-10% the nominal value.
The instrument is planned only for internal use and up to 2000m o.s. altitude.
Before instrument starting up (after taking it from storage), it is suggested that the instrument reaches environmental conditions
(about 30 min).
Storage and transport temperature range: Da +10°C a +40°C
Storage humidity: 80%
Working temperature range: Da +10°C a +40°C
Picture 1 – Registration plate

8
Safety information
This manual contains many information for user safety.
This chapter has been designed and tested in conformity with safety rules.
This manual, if necessary, must be integrated with the provisions on prevention and environmental protection in force
in the country of the operator
Adopted symbols and their meaning
Danger – The danger instructions signal situations that might cause death or serious lesions
Caution - The caution instructions signal situations that might cause physical lesions to people or things
Biohazard – The biohazard instructions signal situations that might cause the contamination of people or parts involved in
the process
Heated surface hazard – heated surface instructions signal surfaces that get hot during use. Risk of burn, avoid direct
contact.
Instructions of danger
Never disassemble, modify or repair the instrument or one of its part without written authorization by Diapath S.p.A.
Do not use any kind of different power supply from the mentioned ones.
Attention to the use of solvents and reagents.
To avoid instrument damages or risks for the user, use reagents indicated in the chart 1.8.1.
Reagents used for tissue infiltration are toxic, flammable and also harmful. Use gloves and protective glasses always.
Gloves must be resistant to all reagents indicated in chart list 1.8.1.
For a correct use of reagents, please refer to their technical data sheets
During processing, do not replace reagents; it may cause damages to the instrument, processing specimens and operator
health too.
All chemical products used in combination with the instrument are highly flammable and may have harmful effects on
health.
Install the instrument in a well-ventilated place and open flame free. People can not stay in the area for many time;
otherwise, a ventilation system is necessary. Do not use in explosion-hazardous areas.
Instructions of caution
Do not place objects that are not involved in the use of the instrument
The use of specific individual protection device is recommended (for instance: gloves)
Replace reagents once exhausted or contaminated
For the disposal of exhausted reagents, please comply with current rules of the company/State where the instrument
is used

9
Instructions of Biohazard
Keep attention to the possible bio-contamination of the parts involved in the process. Follow all decontamination
instructions present in the instrument before any maintenance activity or place the instrument into its case.
Special individual safety measures shall be used by operators, in accordance with the standards for safety in the
laboratories and product safety data sheets in relation to current rules.
Instructions of Heated Surface hazard
Keep attention to liquid paraffin or during racks picking. Liquid paraffin is hot and can cause severe burns.
Do not touch paraffin containers or wax chamber sides because may be hot.
Follow all alerts present next to heated surfaces
Safety training
All the operators must be trained to use the tissue processor safely.
After such training the operators must have understood that:
·The instrument must be connected to a voltage source in accordance with electrical data label
·The use of the instrument differently from Diapath S.p.A. instructions might compromise the supplied protections
Users group
Donatello
TM
tissue processor must be used only by qualified and well-trained operators.
All users can proceed with the use of the device only after reading this user manual and when all technical details are familiar to
them.
Compliance with safety rules
All safety related regulations, local codes and instructions that appear in the manual or on equipment must be observed to ensure
personal safety and to prevent damage to either the instrument or equipment connected to it.
If equipment is used incorrectly, the protection provided might be compromised.
Definition of further adopted symbols
In vitro diagnostic medical device
Built in
Important information for the user
Diapath innovation
European Community approval
mark
Manufacturer
Read carefully the manual before
instrument starting
Danger
Electrical shocks risk
See the user manual
Disposal

10
Definitions
DAF: Decreasing aging factor is a reagent management system according to the number of cassettes processed. Its aim
is to ensure the best use of reagents with an optimal processing.
According to RMS, a limit of usage for each reagent is established which corresponds to the maximum number of
cassettes processed. This limit set by the manufacturer. The system automatically assigns the number of cassettes
processed for each reagent and updates the datum at each processing. This datum resets at reagent replacement
only. The real counter is the DAF (it allows reagent replacement request). DAF factor is not evaluated according to
the number of cassettes processed but in relation to the sequence of reagent used among a group.
Example:
3 tanks of alcohols and 100 cassettes processed during each processing, DAF regression percentage will be equal to
1 – 0.6 – 0.4 (other values 0.3 – 0.2 – 0.15 – 0.1 – 0.1 – 0.05).
After the first processing, the number of cassettes processed set by DAF will be equal to:
100 for the first used tank, 60 for the second and 40 for the third. The system uses the older tank for the first step,
the intermediate for the second and the other for the last step. Tank order use is not fixed and definitive, but
changes when reagent replacement requests occur.
Assign the most recent tank to the last step; it is possible to replace the reagent at any time without waiting for any
DAF indications or in case of unexpected events, for i.e. loss of liquid from a tank. Manual reset, from RMS menu.
EWD: External wax dumping, external wax drain into any container. Exhausted or to replace wax is conducted (using the
specific button) into the processing chamber, and from this last to EWD green connector, placed on instrument
frontal part.
FPS: Fast Processing System: a device that heats the reagents
Multisense: Technology that allows the instrument parameters is to be set automatically according to routing lab needs. There
are 5 preset adjustable profiles set by Diapath Service staff only during installation with the possibility to modify
them in case of routine increase/decrease. Processing operation and the number of available racks inside the
chamber differ from the set mode and the volume of reagent in the tanks.
1. HIGH procedure:
a) Specific for high routine labs
b) MAX 405 specimen
c) RFID active
d) Up to 3 racks with 135 specimens each used at the same time
e) Tanks: 5 L
f) FPS option active
g) Use of standard reagent as xylene and alcohol
2. LOW procedure:
a) Specific for low/medium routine lab
b) MAX 270 specimens
c) RFID active
d) Up to 2 racks with 135 specimens each used at the same time
e) Tanks: 4.1 L
f) FPS option active
g) Use of standard reagent as xylene and alcohol
3. GREEN HIGH procedure:
a) Specific for high routine labs
b) MAX 450 specimens
c) RFID active
d) Up to 3 racks with 135 specimens each used at the same time
e) Tanks: 5 L
f) FPS option disable
g) Use of reagent as Ottix Plus and Ottix Shaper
4. GREEN LOW procedure:
a) Specific for low/medium routine lab
b) MAX 270 specimens
c) RFID active
d) Up to 2 racks with 135 specimens each at the same time
e) Tanks: 4.1 L
f) FSP disable
g) Use of reagent as Ottix Plus and Ottix Shaper
5. OPEN procedure:
a) OPEN profile of the instrument depending on customer needs
b) Possibility of use your own reagents
c) MAX 405 specimens
d) RFID disabled
e) Up to 3 racks with 135 specimens each at the same time
f) Remote Fill and Drain active

11
g) Tanks: 5 L
h) FPS active
Possibility to use any kind of reagent at User’s discretion
PWD: Protected Wax Dumping. Use EWD or PWD mode to drain wax present inside melting cylinders. Liquid wax goes to
processing chamber and then out through EWD connector (green); use protecting mode avoiding leakages of
possible volatile substances present in exhausted wax. This protect procedure conducts the flow to PWD tank (top
right), always empty and specific for exhausted wax drain
RDRAIN: Reagent drain using RFILL connector (red) to an external container using a specific pipe (Available only with OPEN
multi-sense profile)
RFILL: Reagent loading using RDRAIN connector (white) to an external container with its specific pipe (Available only with
OPEN multi-sense profile)
RMS: Reagent management system, is a software page for setting aging limit of single reagent throughout DAF or
process number
SPC: Specimens Processing Chamber (resistances and optical level sensors)
WCS: Wax-cleaning system is a system for wax cleaning from solvent or contamination of alcohols. An airflow allows their
removal.
WWC: Wax Warm Chamber
SelfCheck: SelfCheck is a self-diagnostic system of instrument sensible parts; it prevents unexpected blocks during overnight
processing. The user can activate the procedure before processing:
-Check on the operation of sensible mechanical parts
-Patency test on all reagent position
-Alert in case of malfunction that allows to check the instrument before start processing for a complete safety
of specimens

12
Unpacking and operation
Instrument placement and installation
Requirements for instrument placement
Before proceeding with instrument installation, check if:
·Power sources complies with data supplied by the manufacturer (see Technical Data)
·Place the instrument in a flat, stable and vibration free surface that can carry twice of instrument weight (see Technical Data)
·Make sure that the instrument is level in length and width
·The distance from possible walls shall be at least 20 cm
Delivery
Unpack all instrument parts taking care to not damage them
The instrument is tested before shipment
Check the presence of possible damages due to transport on packaging or instrument. In case of damages, contact Diapath S.p.A. or
local distributor
Unpacking
BOX
OPENING
·To remove the instrument from its packaging
box, open the frontal panel always maintaining
it in vertical position
·Use a screw gun and remove all fixing screws
from frontal panel (Picture 2)
·Pay attention to central screw present on the
bottom
Picture 2 – Box opening
ATTENTION
Check that the instrument case is always in a vertical position (including delivery and unpacking step).
NEVER place the instrument in horizontal position, in order to avoid damaging it and/or its accessories.
ATTENTION
·Due to its dimension and weight (at least 336 Kg), instrument moving shall be performed by several persons
using suitable lifting devices
·Make sure that the instrument is placed and unpacked in a surface not less than 350 cm x 90 cm

13
INTERNAL FIXING PARTS AND ACCESSORIES REMOVAL
Once the frontal cover of the box has been removed, inside the instrument is as shown in Picture 8:
To remove the instrument correctly, proceed as follow (see Picture 3):
·Remove frontal protection black foam rubber (point 1)
·Remove corner protection present on sides, frontal and back of the instrument (point 2)
·Remove polystyrene box containing instrument racks (point 3)
·Remove the box containing fill/drain pipes and paraffin external drain (point 4)
·Remove the box containing 15” PC/monitor and cables (point 5)
Picture 3 – Inside the box
5
3
1
2
4
ATTENTION
·If the instrument is not used immediately, please leave it inside its original packaging.
Remove it only before instrument installation
·Unless different orders, store the box and all inner packaging during instrument warranty
ATTENTION
·Donatello
TM
tissue processor functioning is not allowed in hazardous areas
·Keep the instrument away from heat source as radiators, stoves and direct sunlight; and humidity sources as
basins, drains, etc.

14
After removing corner and frontal protections and all boxes containing accessories, the
instrument is as in Picture 4.
Remove foam rubber protections and the plastic packaging that cover the instrument.
Unlock the instrument (see detail Picture 4) and use panel frontal box as ramp.
Picture 4 - Detail
Apply a light pressure in the area and in the direction showed by the arrow, in order to
unlock the wheel brake (Picture 4A and Picture 4B)
RAMP SETTING UP AND INSTRUMENT REMOVAL
Use box frontal panel as ramp to move the instrument outside.
Place the ramp as shown in Picture 5, ensuring that the two parts (ramp and box lower part) completely fit. Now it is possible to move
the instrument outside (Picture 6).
Lock again the instrument by wheel brakes once located in its final position (see Picture 4A/4B).
Picture 4 – Inside Donatello
TM
Box
Picture 6 –Move the instrument outside
Picture 5 – Ramp and box lower part setting up
Picture 4B - Wheel brake off
Picture 4A – Wheel brake on
ATTENTION
Once unpacked, check both the external conditions of the instrument and the PC. In case of damage DO NOT plug
the instrument to the mains, please contact the m
anufacturer.

15
PC/MONITOR UNPACKING AND INSTALLATION
Instrument PC/Monitor is inside instrument box (Picture 3, point 5). Its packaging is shown in Picture 7:
Remove fixing elements and PC plastic packaging taking care not to cause damage to it.
Mount the PC on instrument supporting bracket as follow:
1. Open support safety lever of the instrument bracket (Picture 8)
2. Place the PC, ensuring its junction with instrument support safety lever is locked correctly (Picture 9)
3. Close safety lever and ensure that the Pc is locked in a correct position (Picture 10)
Picture 7 – Packaging
Picture 8 – Support safety lever Picture 9 – Pc support unlocked Picture 10 – Pc support locked

16
Pc installation and instrument starting up
After fixing the PC to support bracket connect it to the instrument. All cables are installed (it is not necessary to connect them physically
to the instrument because they are already connected internally).
Here below (Picture 11) PC lower part with the list of all connections for instrument functioning:
1 – PC power cable
2 – USB ports for frontal USB port and RFID antenna connection
3 – RJ45 port for communication Ethernet cable and the instrument connection
4 – RJ45 port for instrument connection to local network
INSTRUMENT POWER CONNECTION AND STARTING
Once all cables have been correctly inserted on PC lower part, connect 16A power cable for instrument starting up (Picture 12):
·Ensure that 16A power cable is correctly inserted into instrument socket
·Ensure that the red Voltage Selector, located just above the instrument electrical socket, is set to the correct
position (115V or 220V) in agreement with the local supplied electric voltage (Picture 12)
·Insert cable extremity to an outlet
·To start up the instrument, switch from 0 to 1 (Picture 12)
ATTENTION
·Make sure that the red Voltage selector, located just above the instrument electrical socket, is set to the correct position
(115V or 220V) in agreement with the local supplied electric voltage. Using a higher voltage than the one set on the
selector may cause instrument damage
·Connect the instrument to grounded socket. DO NOT use extension cable
·Use power cable to disconnect the instrument from mains. Check if power cable is easy to access
Picture 11 – Instrument rear part
Picture 12 – Power cable
1
3
Picture 11 - PC rear part
4
2
IMPORTANT
The only cable to plug in is the 16A instrument power supply. All cables are internally connected, the user only have
to connect them to the PC.

17
Re-packing
When repacking due to instrument moving or uninstallation, proceed as follow:
·Remove, if present, the clean basket of processing chamber
·Remove the paraffin from storage reservoir following drain wizard (inner and external) doing a standard cleaning procedure
(see chapter 2)
·Empty all reagent reservoirs (processing, cleaning and paraffin drain)
·Empty the condensation trap (see chapter 2)
·Unplug all cables connected to PC and secure them to the bracket as shown in Picture 13:
·Insert all accessories and other parts as PC, fill/drain pipes and basket into their original packing and seal them carefully
·Place the instrument inside the box: use box frontal wall to create a ramp and allow instrument movement inside the box (see
the procedure RAMP SETTING UP AND INSTRUMENT REMOVAL described before)
·Block instrument wheels
·Place frontal and corner protections inside the box taking care to rear corner ones (see Picture 14)
·Place PC and accessories boxes on the processor, place original packing between instrument and PC’s box
·Close box frontal panel by fixing it with screws
Pay attention to the possible bio-contamination of the parts involved in the process. Before maintenance activity or
place instrument into its case, follow all decontamination instructions present in this manual.
Special individual safety measures should be used by operators, in accordance with the standards for safety in the
laboratories and product safety data sheets in relation to current rules.
Transport
Useful tips for transport:
·
It is scientific instrumentation, it is fragile
·
The instrument has electronic parts
·
Avoid any contact with water and/or other liquids
·
Transport and storage temperature MUST not exceed the following limits: from 5°C to 40°C.
·
Move the instrument only using original packing and keep it always in vertical position. During the transport, special precaution
must be taken in order to avoid instrument overturning.
Picture 13 –Side foam rubber Picture 14 – Instrument bracket

18
Starting-up procedures
Follow these procedures at processor first start-up:
·
Check if power cable is in good condition and connect to both extremities correctly
·
If UPS equipped: check its right functioning (see UPS user manual)
Perform these procedures as soon as instrument switching on:
·
Check system time and date
·
Plan and/or check configuration parameters
·
Plan and/or check all parameters of reagent management
·
Plan and/or check tanks contents
·
Refill wax containers
·
Install reagent tanks
·
Plan and/or check processing protocols
·
Plan and/or check cleaning protocols
Procedure to follow before starting processing
Before starting a protocol, the user must follow the following steps:
·
Check the level of paraffin and reagent containers
·
Check the correct paraffin liquefaction
·
Check and clean processing chamber
·
Select processing program
·
Set all cassettes and racks to process
·
Open processing chamber and insert the rack with the specimens
·
Close processing chamber cap, make sure that is tightly closed
·
Set time and date of ending process
·
Start the processing
Procedure to follow at the end of processing
At the end of protocol, the operator must:
·
Follow the instruction to drain the last reagent
·
Wait for complete drain before opening processing chamber
·
Remove all specimens from processing chamber
·
Clean the processing chamber and its cap from possible residuals of paraffin
·
Perform washing
·
Once washing complete, check if processing chamber and its cap are well-cleaned, if necessary complete the cleaning by
removing any presence of paraffin
·
Check if optical sensors are well-cleaned, if not, clean them using a soft cloth
·
Consult the graph and all statistics of the last processing in order to check if the processing has been accomplished correctly
Procedure to follow in case of instrument switch off
·
If the user has to switch the instrument off, please folllow the instruction below:
·
Access instrument Main menu
·
Access “Diapath settings” menu
·
Select “Exit program” tab
·
Select Exit
Donatello
TM
Series 2 must be switch off ONLY following the procedure described above. In other
case, many damages can involve the instrument and/or data losing
If Donatello
TM
Series 2 is switched off for a long period or must be moved, it is important to
accomplish chamber cleaning and instrument decontamination procedure before its switching off

19
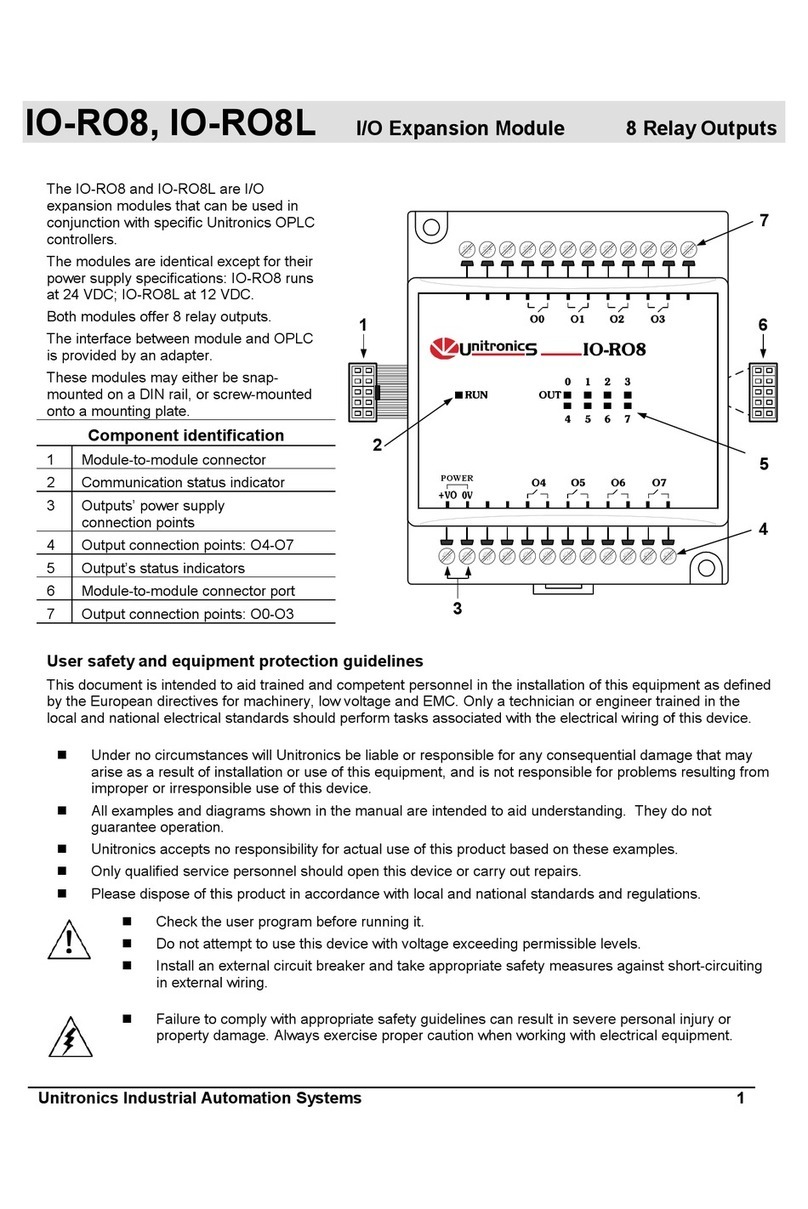
1 DonatelloTM Tissue Processor components
1.1 Instrument overview
1. 15” Color PC panel touch screen 2. Wax chamber WWC
3. Lever SPC door 4. Processing chamber (SPC)
5. RFID reader (for tanks code) 6. RFILL-RDRAIN connectors
7. USB port 8. Tanks compartment
9. Lock – Unlock Lever SPC door 10. EWD
11. PWD 12. P1 – P2 Washing tanks
Picture 15 – Instrument’s frontal view
12
1
1
7
4
3
10
6
1
2
5
9
8

20
1.2 Instrument rear part
Picture 16 shows instrument rear part:
13. Instrument power supply 14. Fuses connectors
15. LAN Ethernet port for PC panel connection 16. RJ12 connector for dialer remote alarm
1.3 Instrument features
·Donatello
TM
tissue processor is an automatic smart tissue processor with vacuum/pressure functions and controlled heating of
reagents and paraffin. It optimizes the use of reagents in order to obtain and maintain an excellent quality of processing
·Maximum loading of 405 cassettes for each run and possibility to set different loading: 270 standard cassettes (2 stainless
steel racks with 135 cassettes each, one above the other) or 405 standard cassettes (3 stainless steel racks with 135 cassettes
each, one above the other), reagent quantity control according set loading
·PWD function (Protected Wax Dumping) for manual or automatic drain of exhaust wax in a specific tank
·EWD function (External Wax Dumping) for manual or automatic drain of exhaust wax through insulated pipe
·9 stations for tanks with processing reagents
·2 stations for tanks with cleaning reagents
·3 basins for wax melting
·1 station for internal drain into wax tank
·Detection of reagent tanks through RFID technology, control on reagent type, batch, expiry date, use and tracking
·SelfCheck: SelfCheck system for the first instrument check components to avoid possible stops during overnight protocols
·Tracking of processed specimens: By an external barcode reader, it is possible to integrate all information of processed
blocks (equipped with a 2D barcode), in order to have a complete tracking of fill/drain phases
·Adjustable temperature range for wax basins; to use wax with melting point between 52-54°C up to 56-58°C
·Visual processing graph with temperature and vacuum/pressure for each processing step
·Possibility to data downloading by USB key
·Processing with non-toxic reagents (Ottix Plus e Ottix Shaper) or with standard protocols (alcohol and xylene or substitutes)
·High-performance charcoal filter to remove harmful vapors with expiry date control
·Filter with suction fan to remove harmful vapors of processing chamber (starting up by the user)
·Automatic system for purification and cleaning of wax (CWS - Cleaning Wax System)
·Reagent pre-heating function, FPS (Fast Processing System) for fast processing of small biopsies
·MultiSense Technology: Automatic setting of instrument parameters based on lab routine needs, 5 default profiles set
during installation and programmable according to routine increasing/decreasing
·Possibility to set a default or customizable end of process by maintaining all specimens inside the first reagent for all delay
length
·Reagent replacement guided by the software, it assists the user in all steps
·Automatic management of reagent exhaustion level with visual alert in case of replacement
13
14
15
16
Picture 16 – Instrument rear part
Table of contents