DigiCare IP41 Series User manual

Infusion Pump IP41x Series
Operator’s Manual
Please read this Operation Manual carefully and follow Precautions for Use
before using the IP41X Series Infusion Pump.
Version 1.1
DIGICARE ANIMAL HEALTH

DIGICARE ANIMAL HEALTH
The intellectual property right of this product and its Manuals belongs to DIGICARE
BIOMEDICAL TECHNOLOGY INC (hereinafter short as DIGICARE).
©2015-2016 All rights reserved DIGICARE BIOMEDICAL TECHNOLOGY INC.
Without prior approval from DIGICARE in writing, this Manual shall not be photocopied, modified
or translated, fully or partially, by any individual or organization.
Statements
DIGICARE reserves the right for final interpretation of this manual.
DIGICARE reserves the right to modify the contents of this manual.
The modified contents should be reflected in the newly published manual version.
This manual is applicable to IP41x Series infusion pumps.
DIGICARE is responsible for safety, reliability and performance of this equipment only in the
case that:
⚫Use in accordance with the Operation manual.
⚫All disassembly, replacement, test, modification and repair are executed by qualified
persons approved by DIGICARE.
⚫All replacement parts, supporting accessories and consumables during the maintenance
are provided by DIGICARE.
⚫Maintenance records for product are reserved.
DIGICARE ANIMAL HEALTH
Thank you for using the IP41x infusion pump from Digicare.
All products manufactured by Digicare Biomedical Technology Inc. are warranted to be free from
defects in material and workmanship and to operate within published specifications, under normal
use, for a period of one year from date of original shipment. The warranty on the Digipump series
is 1 year parts and labor.
The warranty will be void if the pump is altered or repaired or has been misused in any way that
would affect the reliability or detract from the performance.
If you have any questions regarding the IP41x please contact your local distributor or directly
contact DIGICARE.
DIGICARE BIOMEDICAL TECHNOLOGY INC
107 Commerce Road, Boynton Beach, FL 33426, USA.
Phone: (561) 689-0408
Fax: (561) 689-0021
Website: www.digi-vet.com
Sales email: sales@digicarebiomedical.com
Technical Support email: techsupport@digicarebiomedical.com

Contents
1OVERVIEW .......................................................................................1
1.1 INTENDED USE ....................................................................................................1
1.2 CONTRAINDICATION ..........................................................................................1
1.3 PRODUCT FEATURES...........................................................................................1
2PRECAUTIONS FOR USE .................................................................3
3PRODUCT SPECIFICATIONS...........................................................8
4PRODUCT DESCRIPTION............................................................. 11
4.1 PRINCIPLE OF OPERATION ..............................................................................11
4.2 COMPOSITION OF INFUSION PUMP ...............................................................11
4.3 HANDLE ...........................................................................................................14
4.4 DROP SENSOR .................................................................................................14
4.5 POLE CLAMP....................................................................................................14
4.6 NURSE CALL ....................................................................................................15
4.7 ACCESSORIES ACCOMPANIED .........................................................................15
4.8 OPTIONAL ACCESSORIES ................................................................................15
5PREPARATIONS FOR USE............................................................ 16
6OPERATING INSTRUCTIONS ...................................................... 17
6.1 DISPLAY AND KEYS ..........................................................................................17
6.2 START UP .........................................................................................................19
6.3 CALIBRATION OF NEW IV SETS ........................................................................20
6.4 IV SET INSTALLATION……………………………………………….23
6.5 PURGE…………………………………………………………………..24

Contents
6.6SETTING THE INFUSION RATE………………….……………………………………...25
6.7 PUNCTURE……………………………………………………………..…………………26
6.8 STARTING INFUSION……………………………………………………………………26
6.9 CHANGE RATE DURING INFUSION…………………………………..……………….26
6.10 BOLUS……………………………………………………………………………………..27
6.11 STOPPING INFUSION……………………………………………………………………28
6.12 REPLACING OR ADJUSTING IV SET………………………………..…………………28
6.13 TURNING THE POWER OFF…………………………………………..29
7 INFUSION MODES………………………………………………………..30
7.1 INFUSION SET...................................................................................................31
7.1.1 Infusion Mode…….……………………………………………….30
7.1.2 Occlusion Level……………………………………………………33
7.1.3 Bolus Mode......................................................................................33
7.1.4 KVO Rate.........................................................................................34
7.1.5 Brand................................................................................................33
7.1.6 Relay set...........................................................................................34
7.1.7 Drip Mode Set..................................................................................34
7.1.8 Micro Mode Set ...............................................................................35
7.1.9 Air bubble testing level....................................................................35
7.1.10 Near Finished...................................................................................35
7.1.11 Recent Therapy................................................................................35
7.2 LOCAL SET .......................................................................................................36
7.2.1 Volume Setting ................................................................................36
7.2.2 Display SET.....................................................................................36
7.2.3 Internet Set.......................................................................................37
7.2.4 Lock screen Set................................................................................38

Contents
7.2.5 Collection Set...................................................................................40
7.2.6 Linkage mode...................................................................................40
7.2.7 Pressure Unit....................................................................................41
7.2.8 Date & Time set...............................................................................41
7.2.9 Maintenance.....................................................................................41
7.3 HISTORY...........................................................................................................42
7.4 PATIENT FILE....................................................................................................43
7.5 USE INTERNAL BATTERY ..................................................................................43
7.6 CONNECTING TO THE <INFUSION CENTRAL MONITORING
SYSTEM>(OPTIONAL)..................................................................................................45
7.7 NURSE CALL (OPTIONAL) ................................................................................45
7.8 VOICE COMMUNICATION(OPTIONAL) ............................................................45
7.9 CONNECTING A BARCODE SCANNER (OPTIONAL) .........................................45
7.10 USER-SPECIFIC REQUIREMENTS (OPTIONAL) .................................................45
7.10.1 Maximum Flow rate.........................................................................45
8TROUBLESHOOTING.................................................................... 46
8.1 ALARM .............................................................................................................46
8.2 FAULTS AND TROUBLESHOOTING...................................................................47
8.3 TROUBLES AND TROUBLE SHOOTING .............................................................48
9MAINTENANCE ............................................................................ 49
9.1 CLEANING,DISINFECTING...............................................................................49
9.2 PERIODIC MAINTENANCE ...............................................................................49
9.2.1 Checking the Appearance................................................................49
9.2.2 Checking the Power Cable...............................................................49

Contents
9.2.3 Checking the infusion rate...............................................................50
9.2.4 Alarm ...............................................................................................50
9.2.5 Electric and mechanical safety.........................................................50
9.2.6 Checking the Internal Battery..........................................................50
9.2.7 Replacing the Battery.......................................................................50
9.3 MAINTENANCE ................................................................................................51
9.4 STORAGE..........................................................................................................52
9.5 TRANSPORTATION...........................................................................................52
9.6 ENVIRONMENTAL PROTECTION AND RECOVERY...........................................52
10 INFUSION ACCURACY CHARACTERISTICS................................ 53
10.1 FLOW RATE CHARACTERISTICS.......................................................................53
10.2 OCCLUSION CHARACTERISTICS ......................................................................56
APPENDIX A ELECTRON MAGNETIC COMPATIBILITY (EMC) ........... 57
APPENDIX B THE DEFAULT FACTORY SETTINGS ............................... 63
APPENDIX C TOXIC AND HAZARDOUS SUBSTANCES OR ELEMENTS
64
APPENDIX D DIFFERENCE BETWEEN IP41X SERIES ........................... 65

Overview
1/ 63
1Overview
1.1 Intended use
This product is intended for the administration of fluids to patients requiring continuous
delivery at controlled infusion rates and should only be used by trained professionals.
1.2 Contraindication
This product is not intended for blood transfusions.
1.3 Product Features
DIGICARE IP41x Series is a micro-continuous infusion pump. It ensures constant
infusion speed and accurate dosing volume during long time infusion.
This infusion pump is used for continuous and micro-volume infusion of liquid or liquid
medicine of small volume and high concentration, including, but are not limited to the
infusion of chemotherapeutic agents, cardiovascular drugs, antineoplastic, oxytocic,
anticoagulant, anesthetic agents.
⚫Standard disposable IV sets are supportable.
⚫User can include new standard IV set.
⚫Eleven occlusion levels and displays the pressure status of the IV line.
⚫Maximum infusion rate can be set to 1200mL/h.
⚫Calibration function for infusion accuracy is available.
⚫Safety design by monitoring infusion states.
⚫Multiple modes of infusion.
⚫Multi-channel infusion workstation relay infusion function.
⚫WI-FI function, can be connected to the infusion central monitoring system by
intravenous infusion.
⚫Touchscreen, providing quick and convenient user interface.
⚫Night mode display, reducing light interference to patients and environment
⚫Three types of power supply: AC mains supply, DC external supply, and internal
lithium battery. The internal lithium battery can power the infusion pump for no less
than 5 hours (at 25ml/h rate).
⚫Dual CPU and redundancy design for increased safety.
⚫Two-way alarm for monitoring the main control circuit and motor drive circuit.
⚫Independent motor driving CPU and motor subdivided drive chip design.

Overview
2/ 63
⚫Setting for automatic prompt of maintenance interval.
⚫Modular installation design enables multi-channel pumps among pumps.
Note:
Drip sensor, Barcode scanner, WI-FI communication module, voice communication,
nurse call and relay infusion function are optional.

Precautions For Use
3/ 63
2Precautions for Use
In this manual, precautions are classified into warning and caution as follows:
WARNING:
A Warning alerts you to a potential serious outcome, adverse event or safety hazard.
Failure to observe a warning may result in serious injury.
CAUTION:
A Caution alerts you to where special care is necessary for the safe and effective use of
the product. Failure to observe a caution may affect normal use of the product.
WARNING
⚫The infusion pump must be operated by qualified clinical professionals.
⚫The infusion pump cannot be used for blood transfusion.
⚫The pump does not have an upstream occlusion alarm, clamps must be below the
pump at all times.
⚫Prior to use, please check the status of the pump, power cord and other related
accessories to ensure the device can be used normally and safely.
⚫Pay extra attention to kinks of the infusion line when it is used for low-infusion. The
smaller the set infusion rate becomes, the longer it takes from the occurrence of
occlusion to its detection, which may suspend the infusion for a long time.
⚫To avoid the risk of fire or explosion, do not use the infusion pump in a flammable or
oxygen rich environment.
⚫The altitude difference between the pump and heart position of the patient should
not be larger than 100cm (3.3ft). Smaller difference of the altitude will increase the
accuracy of the pressure sensor’s result.
⚫In the event of tube twisting, filter condensation or intubation occlusion during
infusion, the internal pressure of the infusion tube will increase. Once the causes for
occlusion are removed, too much infusion liquid may be infused into the patient.
Therefore, proper actions should be taken. For example, clamp the infusion tube
before removing the occlusion causes.
⚫To guarantee the infusion safety and alarming function, it is recommended to use the
IV sets specified by the manufacturer only.
⚫Only the IV set, tube, infusion needle and other medical parts complying with the

Precautions For Use
4/ 63
local regulations can be used on the infusion pump. Contact Digicare or your local
distributor for more information.
⚫Operations against the requirements, procedures, warnings and cautions provided in
this manual may cause infusion failure, inadequate, overdosing, or other potential
risks.
⚫It is recommended to install the drop sensor and open the drop monitoring function. A
long time extrusion without moving or replacing the tube may cause an inadequate
infusion.
⚫There should be regular monitoring by clinical professionals to observe the clinical
situation and infusion pump working condition when using the device.
⚫The power cord or other affiliated lines should be kept properly to avoid any risk of
twining on patient or electrical disturbance.
⚫High-frequency surgical equipment, mobile phone, wireless device and defibrillator
may have interference on the infusion pump. Keep away from such devices while
operating.
⚫To avoid the risk of electric shock, this equipment must only be connected to a mains
supply with protective earth.
⚫If the pump and its related accessories are reaching over the lifetime, they must be
scrapped and disposed in accordance with the local laws or hospital ordinances.
Please contact your local representative for further details.
⚫Do not modify this equipment without authorization of the manufacturer.
⚫When operating the pump or checking the pump's alarm system, the operator shall
be in front of the device, no farther than 1 meter.
⚫There is no patient circuit in this device. The output of the equipment is not
allowed to be accessible to patient.
⚫The operator shall not touch IP41x and the patient simultaneously.
CAUTION:
⚫The infusion set is treated as an applied part of the pump.
⚫Check the setting values on the prescription and infusion pump. Infusion should start
only when the values are equal.
⚫In order to prevent extrainfusion, close the rolling clamp of the IV sets before removing
the IV set from pump.
⚫Follow the tube replacement alarm on the display interface, replace the IV sets or
move the IV sets tubing more than 10cm, to keep the infusion accuracy continuously.

Precautions For Use
5/ 63
⚫Ensure that the infusion pump has been fixed tightly on the stand and the stand is
stable. Prevent the pump from collision, dropping mechanical vibration or other impact
of external forces to avoid damage on the pump.
⚫Before pressing the [START] key, check if the infusion speed is correct, especially the
position of the decimal point.
⚫Do not operate on the display with sharp objects. Otherwise, the display may be
damaged.
⚫Occlusion alarm may occurwhen high-viscosity liquid is infused at high speed through
a thin intravenous needle.Increase the occlusion level or decrease the infusion speed.
⚫The drop sensor detects drops, but not the flow rate. If the liquid in the drop chamber
keeps dripping into continuous liquid flow, the drop signal cannot be detected.
⚫Infusion pump should be placed without the reach of patients and other irrelevant
personnel.
⚫Avoid direct sunlight, high temperature and high humidity.
⚫Do not disinfect the infusion pump by using high-pressure steam sterilization
method.
⚫Before using the internal battery, check the battery to ensure that sufficient power is
available. Recharge, if required.
⚫Ensure that the infusion pump always has a battery installed during operation.
Otherwise, the system may stop without issuing an alarm when external power is
interrupted due to power failure or a short circuit, causing an unsafe condition.
⚫If the infusion pump cannot work as described in this manual for unknown reasons,
stop it and report the details (including IV set, infusion flow, serial number of infusion
pump, and type of infusion liquid) to your local distributor or our customer service
department.
⚫Do not disassemble or reconstruct the infusion pump without authorization.
⚫Liquid intrusion into the AC power socket, USB or nurse call socket may cause short-
circuiting. While connecting the power cable, check if the connecting parts are dry. If
liquid spills on the infusion pump, clean the pump with a dry wiper. Use after the
service engineer checking.
⚫The maximum temperature at the applied part of the pump may reach 41.1℃, when
running continuously under the highest environment temperature at the highest
infusion rate.
⚫Before use, carefully check if the occlusion pressure test function of the infusion

Precautions For Use
6/ 63
pump is normal. The Maximum infusion pressure at the end of the infusion tube
generated by the pump may be up to 3500mmHg under the condition of occlusion
when sensor failed.
⚫The delay time between the onset of the alarm condition and the representation of
the alarm is no longer than 150ms.

Precautions For Use
7/ 63
Symbols
Authorized Representative in the European Community
CE Mark: conforms to essential requirements of the Medical
Device Directive 93/42/EEC.
Date of manufacture.
Manufacturer
Specifies serial number
TYPE CF APPLIED PART
Alternating current
Direct current
DISPOSAL: Do not dispose this product as unsorted
municipal waste. Collection of such waste separately for
special treatment is necessary.
CAUTION! Read the accompanying document.
General warning sign
Refer to instruction manual / booklet
IPX2
Level of protection from liquid intrusion
Interference may occur near the devices with below sign.
Nurse call
ON/OFF
HOME
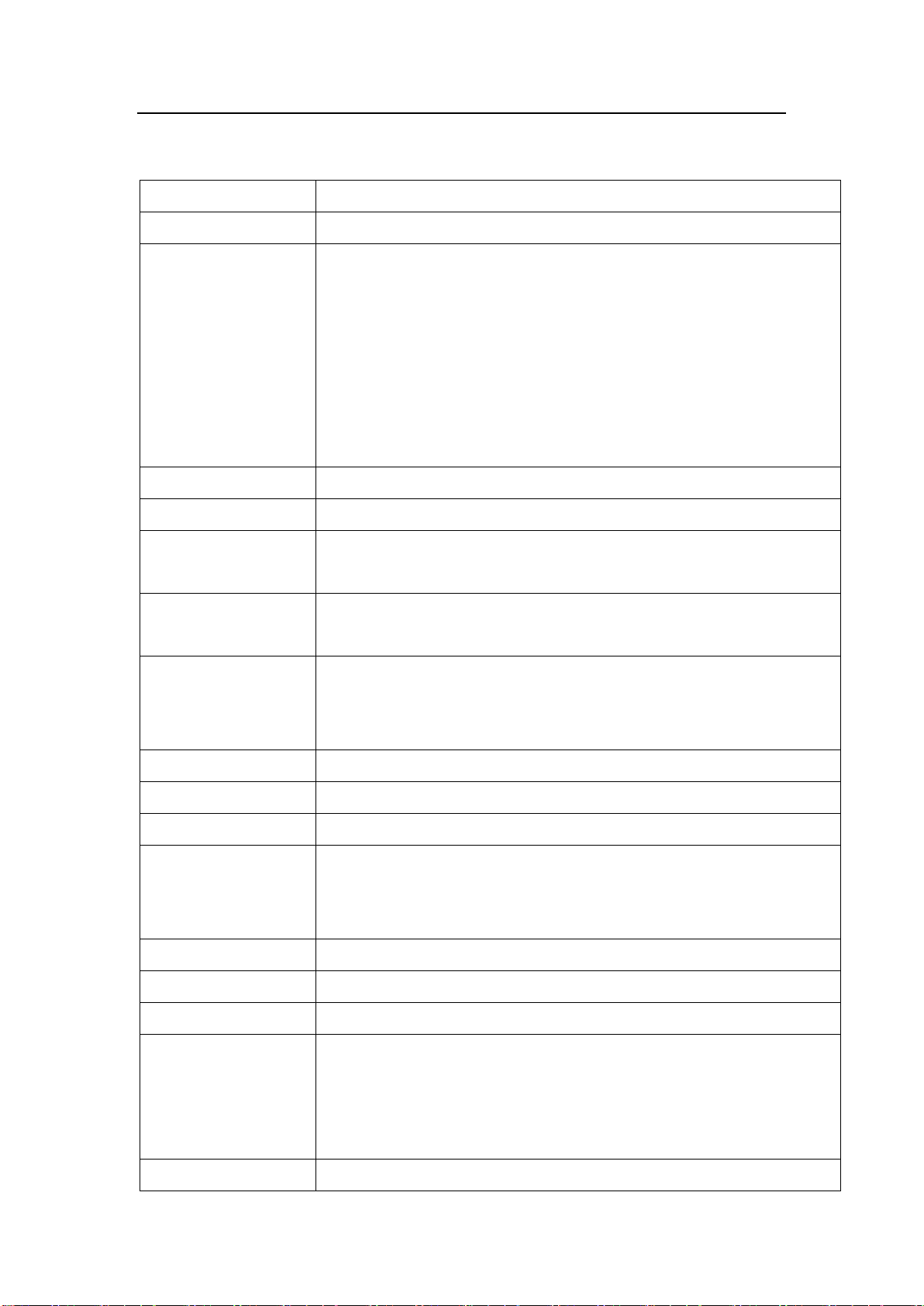
Product Specifications
8/ 63
3Product Specifications
Product name
Infusion pump
Model
IP41x Series
Power supply
AC power supply:
AC 100-240V,50/60 Hz, power consumption 45 VA
External DC power supply: DC 12 V 1A
Internal battery: lithium battery 11.1 V 1500 mAh
Battery model: 154457
Time of battery continuous use: no less than 5 hours (for infusion at 25
mL/h rate with a new battery)
Fuse
T1.6AL 250VAC
Compatible IV sets
IV sets with external diameter from 3.5 to 5.5mm, shore A max 75.
Infusion mode
Rate, Time, Weight, Loading Dose, Trapezia, Sequence, Micro, Relay,
Drip Mode
Infusion setting
range
0.1-1200.0mL/h or(0.03-400d/min)
See the least increment in chart 6-3
VTBI setting range
0.1 - 99.99(Least increment 0.01)
100 - 999.9(Least increment 0.1)
1000 - 9999(Least increment 1)
Total volume display
0-99999.99ml
Accuracy
±5%
Purge operation
1200.0ml/h
Bolus operation
0.1~1200.0ml/h
Automatically calculate the bolus rate by bolus amount, cannot lower
than the current rate.
KVO rate
0.1-5.0mL/h
Air-bubble sensor
Sensitivity: detect air-bubble ≥
Occlusion level
225mmHg~975mmHg, 11 levels are available
Alarm
Near Finished, Finished, OCCL, Low Battery, Battery Empty, No
Battery, No Power Supply, The Pump Door Open, Air Bubble , No Drip
Sensor, No Drips, Drips Abnormal, Reminder Alarm , Relay Index
Duplicate, Infusion Start Fail , Standby Time Expired
Special function
Repeat alarming:
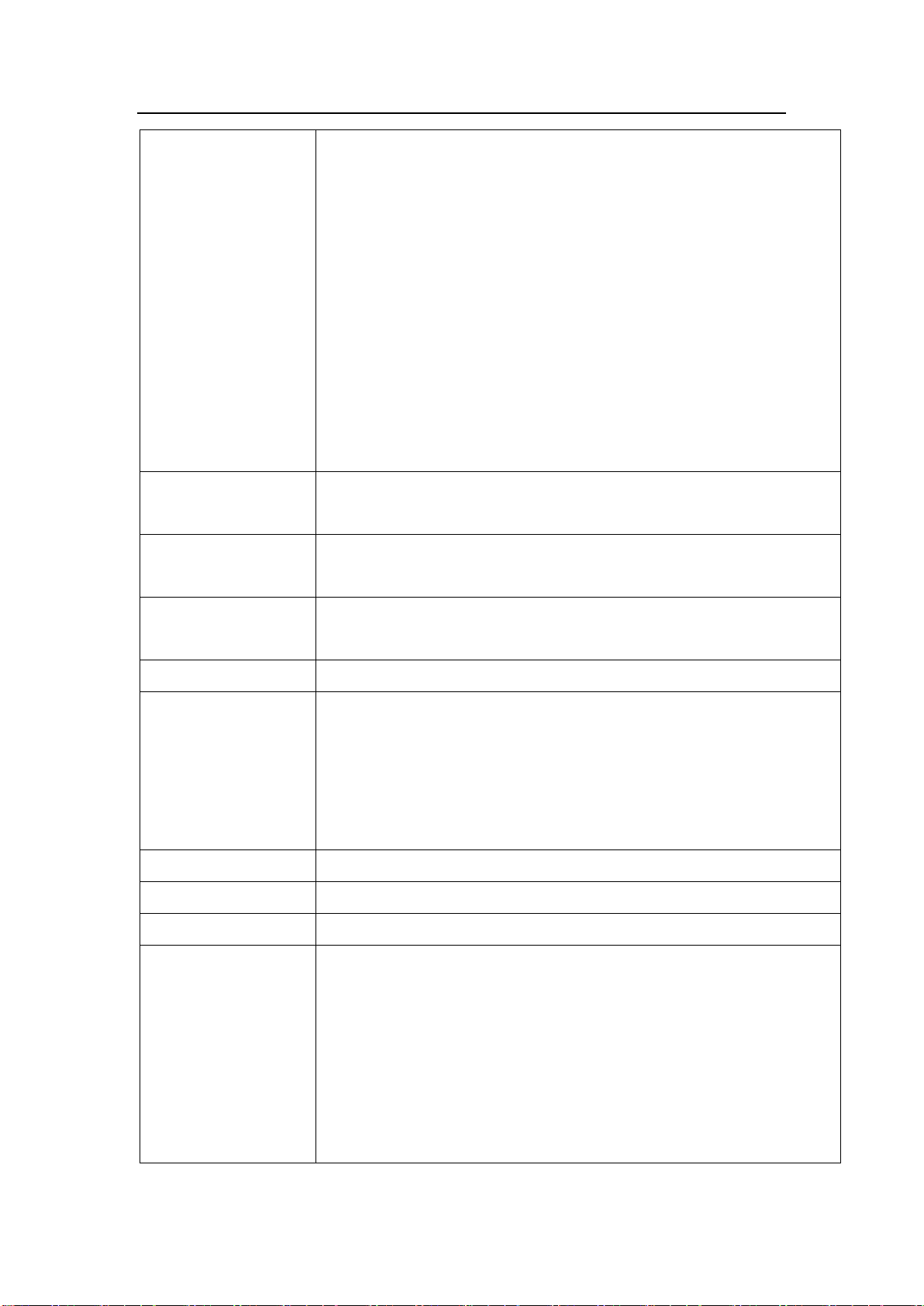
Product Specifications
9/ 63
If there is still alarm after mute alarm sound, it will alarm again in 2
minutes
Event recording:
can store and playback 2000 events maximum
Sound volume:
11 levels are available
Power supply switching:
When AC/DC power supply is cut off, the infusion automatically switch
to internal battery supply
Barcode scanning:
Input the patient information by barcode canning
WI-FI function
Connect infusion workstation, nurse pager, voice communication
and infusion pump information network
Operating
conditions
Temperature: 5℃to 40℃Humidity:15% to 95% RH
Pressure altitude: 70.0kPa-106.0kPa
Storage conditions
Temperature: -20℃to +55℃Humidity: 10% to 93% RH
Pressure altitude:22.0kPa-107.4kPa
Operation Mode
Continuous operation
Classification
1. Class I / Internally powered equipment;
2. Type CF applied part;
3. IPX2;
4. No sterilization requirement for pump
5. Not category AP / APG equipment;
6. Mode of operation: continuous
Dimensions
202(W) ×74(H) ×133(D)mm
Weight
< 1.22 kg (including battery)
Service Life
10 years
Main safety standards
IEC60601-1 Medical electrical equipment - Part 1: General
requirements for basic safety and essential performance
IEC60601-2-24 Medical electrical equipment –Part 2-24: Particular
requirements for the safety of infusion pumps and controllers
IEC60601-1-8 Medical electrical equipment -- Part 1-8: General
requirements for basic safety and essential performance -- Collateral
standard: General requirements, tests and guidance for alarm
Product Specifications
10 / 63
systems in medical electrical equipment and medical electrical
systems
IEC60601-1-2 Medical electrical equipment - Part 1-2: General
requirements for basic safety - Collateral standard: Electromagnetic
compatibility requirements and tests

Product Description
11 / 63
4Product Description
4.1 Principle of Operation
The IP41x Series infusion pump mainly consists of pump shell, display screen and
operating system, monitoring system, alarm system, motor drive system, tubing
peristaltic module, power supply system, drop sensor (optional), WI-FI communication
module (optional), handle and pole clamp.
The infusion pump adopts the dual processor structure, controls the motor precisely,
drives the peristaltic sheet to infuse through the mechanical drive device, monitors the
sensors and infusion process, and provides audio and visual alarms.
4.2 Composition of Infusion Pump
1 –Touchscreen
2 –[HOME] key
3 –[ON/OFF] key
4 –[OPEN] key
5 –pump door
6 –Shell
7 –Alarm indicator

Product Description
12 / 63
1 –Lighting lamp
2 –Depressor
3 –Anti-free-flow clamp
4–Anti-free-flow clamp button
5 –Peristaltic pump plate
6 –Air bubble sensor
7 –Pressure sensor
8 –Infusion tube slit
9 –Catch
⚫Lighting lamp. To provide lighting in a dim environment, so as to install and check
the infusion tube
⚫Depressor and peristaltic plate. Driven by the step motor, press and move the tube
to realize liquid flow
⚫Anti-free-flow clamp. Stop liquid flow and infusion backwards after the pump door
opens
⚫Anti-free-flow clamp button. Press the button and the clamp will automatically open
or close.
⚫Pressure sensor and bubble sensor. Sensors monitor occlusion pressure and air
bubble inside the infusion tube.
⚫Infusion tube slit. At sides of pump to guide the infusion tube in a line behind the
pump door.
⚫Catch. The two catches are used to close the pump door.
Product Description
13 / 63
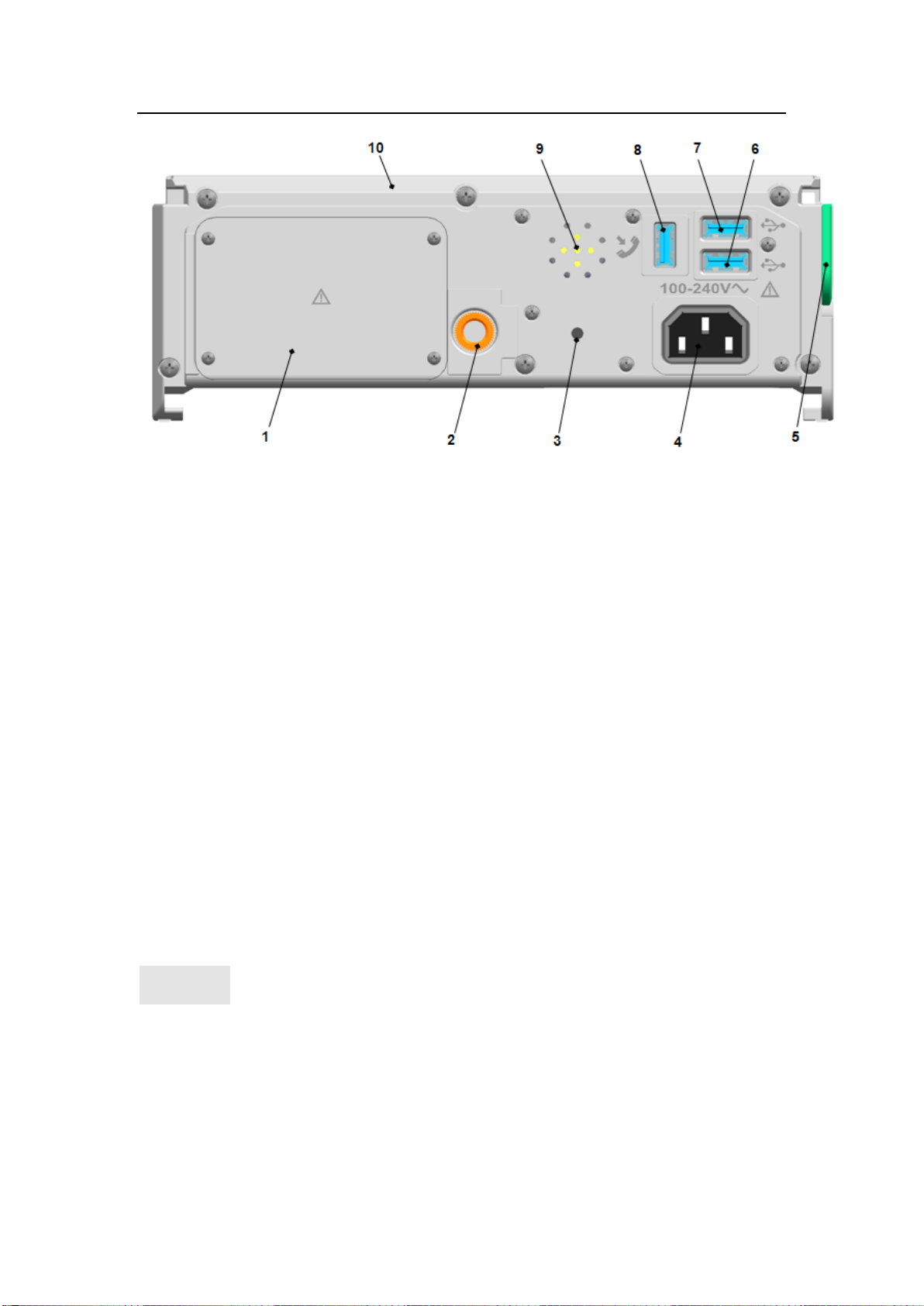
1 –Battery cover
2 –Threaded hole
3 –Auxiliary alarm
4 –AC power inlet
5 –Combination clamp
6 –External inlet 1
7 –External inlet 2
8 –External inlet 3
9 –Buzzer
10 –Shell
⚫Battery chamber. Replaceable battery inside the chamber.
⚫Threaded hole. To fix the pole clamp, then fix the pump to the IV pole via the pole
clamp.
⚫Auxiliary alarm. Audible alarm sounds when product functions abnormally.
⚫Buzzer. To alarm in high, medium or low level during infusion and enable voice
conversation.
⚫AC power inlet. To connect the external AC power source.
⚫External inlets 1, 2 and 3. The three inlets share the same signal and can be
connected to 3 external devices at the same time. The external devices include drop
sensor, barcode scanner, external DC power cord. The external inlet 1 and 2 could
be used as the interface for the local WLAN.
CAUTION:
⚫Only the accessories or devices specified by the manufacturer allowed to be
connected to the Pump. Otherwise may cause electrical shock. See Table 4-1.
⚫The person who connects the devices and accessories to each other or who uses
the devices and accessories is responsible and liable for installation and operation
that complies with IEC/EN 60601-1-1 or clause 16 of IEC 60601-1.
⚫The plug is used as disconnect to the mains supply, do not position the pump so
Table of contents