● Do not use a damaged or broken power cord, plug, or outlet. It may cause fire and/or electric shock.
● When unplugging the power cord, do not touch cord with wet hand.
● Do not use loose power cord, it could cause electric shock and fire.
● Do not damage the power cord (do not bend too hard, or put heavy objects on it).
That may cause electric shock and/or fire.
● Unauthorized modification and dismantling is prohibited.
● Do not use a solvent such as thinner or benzene for cleaning.
※ Please use an absorbent and alcohol to wipe the controller box. Do not wipe with wet cloth.
※ Be careful not to drop liquid on control box.
● Do not place the product near any heated equipment and do not put candles or cigarettes on it.
● Use only a grounded outlet. Please contact electrical technician or manufacturer regarding the ground connection.
● The product must be used by specialist or dentist. If not, patient might get hurt from improper use.
● If patient is taking antibiotics, patient needs to consult with doctor before operation.
● Keep product away from spray containing flammable material.
● Manufacturer does not have any responsibility for defects or loss to property in the following cases:
1. User did not follow the instruction manual when using the product.
2. Used the product in a place of unregulated wire condition.
3. Unauthorized person repaired the product.
4. Did not follow the instruction manual for this product.
● Do not use on the following patients.
1. Those with medical complications or allergies
2. Those who have pre-existing conditions (E.g. Cardiac, Pulmonary, Renal disturbance or High blood pressure)
3. Those who are pregnant or lactating
4. Patients with cardiac pacemakers and infants
PROHIBITED
<Ultrasonic Surgery Functions>
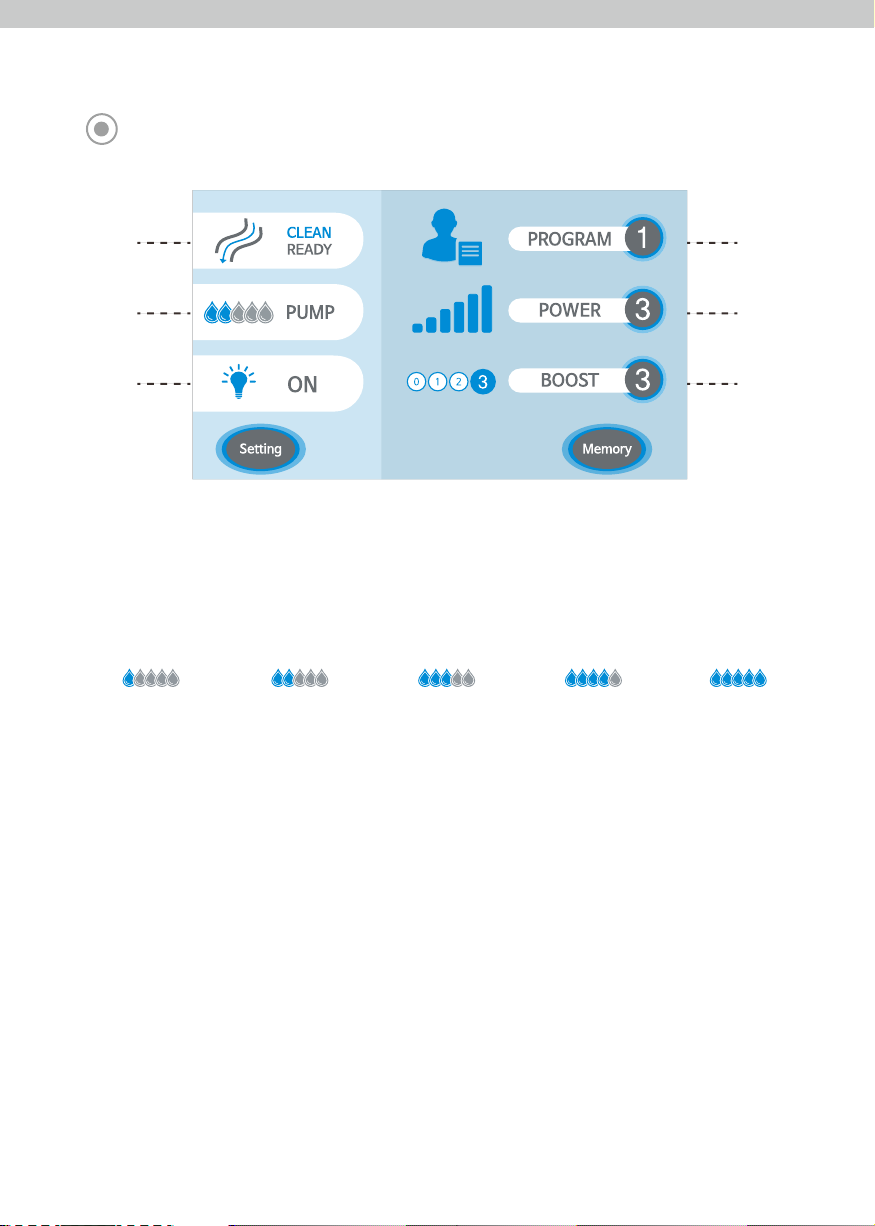
- Users can set up Power and Boost selections in order to have the best settings for each individual.
- Power: Level 1, 2, 3
- Boost: Level 0, 1, 2, 3
<Program Memory Function>
- 3 programmable memories for setting Power, Boost, Irrigation Pump, Optic.
(There are total 5 programs and program no. 4 and no. 5 have certain settings for ultrasonic tips so they have separate
Power and Boost setting.)
<Clean/Ready Function>
- It cleans out the air in the irrigation tube to produce consistent flow before and after surgery.
<Ergonomic Foot Controller>
- The ergonomically designed Foot Controller with key functions can be used during surgery for user’s convenience.
<Optic Function>
- LED light from piezo handpiece helps to see clearly during any surgery.
(It only operates on the piezo handpiece with an optic function.)
PRODUCT FEATURES & ADVANTAGES
4