Envitec PhysioQuant User manual

PhysioQuant
Ambulatory Blood Pressure System
User-Manual
ENVITEC

2 / 48
3 / 48
User-Manual
PhysioQuant Ambulatory Blood Pressure System
The greatest care has been taken in preparing this manual. However, if you still
come across any incorrect details in this manual when using the system, then
please contact us. This will allow us to correct any errors as soon as possible.
The information and images contained in this manual are subject to any
changes that may result from optical or technological developments
All trademarks stated and used in the text are the property of the present owner
and are protected by law.
All reproduction, translation and duplication in any shape or form – including
extracts – require the written approval of the manufacturer.
This manual is subject to updates by EnviteC-Wismar GmbH.
Doc.No.: 45-07-0500002-a © 2004 Envitec Wismar GmbH
(Printed in Germany)
Envitec Wismar GmbH Tel.: +49 - (0) 3841-360-1
Alter Holzhafen 18 Fax: +49 - (0) 3841-360-222
Germany Website: www.envitec.com

4 / 48

5 / 48
Contents
1GENERAL REMARKS / SYMBOLS 7
2SAFETY INSTRUCTIONS AND USE 8
3INSTALLATION 11
3.1 Software 11
3.2 Settings 14
3.3 Attaching the connecting cable to the recorder 17
3.4 Network installation 18
3.5 GDT interface 18
4PHYSIOQUANT RECORDER 21
4.1 Operation controls 21
4.2 Symbols on the device 22
4.3 LCD display with all symbols and display options 23
4.4 Power supply 23
4.5 Inserting batteries 24
4.6 Function control 25
4.7 Connecting the cuff to the recorder 26
5PHYSIOQUANT CUFFS 26
6COMMENCING LONG-TERM BLOOD PRESSURE MEASUREMENT 27
6.1 Connecting the recorder 27
6.2 Starting the programme 27
6.3 Selecting a patient 28
6.4 Adding a new patient 29
6.5 Editing patient data 29
6.6 Programming the recorder 30
6.7 Attaching the cuff and the recorder 32
6.8 Test measurement 33
6.9 Instructing the patient 34
6.10 Important measurement information 35
6.11 Button functions 36

6 / 48
7IMPORTING RECORDER DATA AFTER MEASUREMENTS 37
7.1 Connecting the recorder 37
7.2 Starting the programme 37
8ANALYSIS OF MEASUREMENT RESULTS 38
8.1 Starting the programme 38
8.2 Find/delete measurement results 38
8.3 Representation 39
8.4 Overview 40
8.5 Single-value graph 40
8.6 Single-value table 40
8.7 Hourly mean values graph and table 40
8.8 Findings report 40
8.9 Printing 41
8.10 GDT export to general practice IT systems 41
9ERROR CODES 42
10 SCOPE OF DELIVERY 43
11 ACCESSORIES AND SPARE PARTS 43
12 CLEANING AND MAINTENANCE 44
12.1 Cleaning and disinfection of device surface 44
12.2 Cleaning and disinfection of cuffs 44
12.3 Cleaning of tubes 45
12.4 Maintenance and validity check prior to any use 45
12.5 Calibration mode 46
12.6 Disposing of the device 46
13 TECHNICAL INFORMATION 47

7 / 48
1 General Remarks / Symbols
This symbol means: please consult the
manual. It refers to things to which you
should pay careful attention when using the
device.
The safety instructions in this manual are indicated in the following
way:
This refers to a potentially dangerous
situation. Failure to observe this warning may
result in injuries and/or damage to the
product.
CAUTION!

8 / 48
2 Safety Instructions and Use
•The product PhysioQuant carries the CE mark CE-0123 in accordance
with European Council Directive 93/42/EEC in relation to medical devices
and complies with the fundamental requirements stated in Appendix I of
this Directive. The device has an internal power source and comes within
Class IIa (MDD).
•The device has an application part of the type 'BF'.
•Standard EN 60601-1 'Medical electrical equipment, Part 1: General
Requirements for Safety' is complied with, as well as the immunity
requirements of standard EN 60601-1-2 'Electromagnetic Compatibility -
Medical Electrical Equipment'.
•The device is interference-free in accordance with EN 55011 - Class B.
•The CE mark only includes those components described in the delivery
overview.
•This manual forms part of the device. It should always be kept near to the
device. Correctly observing the manual ensures that the device will be
used in an appropriate manner and for the purpose it is intended. It will
also ensure the health and safety of users and patients dependent on it.
•Please read through the whole manual carefully, since information that is
relevant to several sections is only provided once.
•The printed text of this manual is in accordance with the version of the
device, as well as the relevant safety instructions standards, at the time
that this manual was printed. All industrial property rights are reserved in
relation to any devices, circuits, processes, software programs and names
described in this manual.
•The quality assurance system used by EnviteC-Wismar GmbH in all the
company facilities complies with standards EN ISO 9001 and EN ISO
13485.
•In order to ensure the highest level of safety for patients and a minimum of
interference, as well as keeping in line with the relevant testing precision
level, the device should only be used in combination with original
accessories provided by EnviteC-Wismar GmbH.
•No warranty claims can be made in the event of any damage as a result of
using other inappropriate accessories and consumable materials.

9 / 48
•EnviteC will only assume responsibility for devices in relation to their
safety, reliability and functioning in the following cases:
- Assembly, enhancements, resettings, alterations and repairs carried
out by EnviteC, or at locations that have been expressly authorised by
EnviteC to carry out such repairs;
- Devices that have been used in accordance with their manuals.
•Intended use:
PhysioQuant is a manually-operated blood pressure measuring device,
carried by the patient for long-term measurement of non-invasive blood
pressure. It can be used for adults, children and infants by applying the
corresponding cuffs. PhysioQuant must not be used for newborn babies
and is not suitable for use in Intensive Care Units. PhysioQuant can be
used to take blood pressure measurements at various intervals for up to 30
hours and is able to store measurement results.
•Biocompatibility:
The product components described in this manual, including accessories,
which – when used for their intended purpose – come into contact with
patients, have been explained in such a way that they conform to the
biocompatibility requirements of the relevant standard when used for their
intended purpose. If you have any questions in this regard, then please
contact EnviteC-Wismar GmbH or one of its representatives.
•Cleaning:
No fluid should enter the device. If any fluid has entered the device, then it
should only be used again after having been checked and approved by the
Service Department.
•Only clean the PhysioQuant Recorder when it is not connected to another
device.

10 / 48
•The device is not suitable for use in areas where explosions may occur.
Such areas may appear as a result of using inflammable anaesthetics, skin
cleaning and skin disinfection products.
•The device should only be used together, or in combination with
components of other equipment, when you have ensured that this
connection does not adversely affect the health and safety of patients and
users, or the environment.
•If the information provided with the device is not clear on how to make a
safe connection that will ensure the health and safety of patients, users, as
well as the environment, then please contact the manufacturer or consult a
technical expert. At all times, observe standard IEC 60601-1-1.
•The PhysioQuant Recorder can be connected to, and operated from, a PC
on which PhysioQuantWin software has been installed. Please note that
no patient should be connected to the PhysioQuant Recorder as long as it
is connected to the PC.
•Before using the device on a patient, the user should check that the device
functions safely and as intended.
•Users must be familiar with how to operate the device.
•Medico-technical devices should only be used by suitably-qualified or
experienced persons, who can ensure that the device is used correctly.
•The device does not contain any components that need to be replaced by
users. Never open the casing of the device (please contact the Service
Department).
CAUTION!

11 / 48
3 Installation
3.1 Software
•Put the PhysioQuant CD in your CD-ROM drive.
•If the 'Auto start Function' of your CD-ROM drive is activated, then
installation will begin automatically. If not:
- Open Windows Explorer, select your CD-ROM-drive and
double click on setup.exe.
•The installation wizard appears:
•Confirm that you are to start the installation by clicking on [Next]
and follow the on-screen instructions.

12 / 48
Enter your name and that of your practice and confirm with [Next].
The programme will normally be installed under your standard program
directory. You can change this location via [Browse].

13 / 48
All settings are summarised before installation commences. After
confirming with [Next], the copy and installation process begins.
Once the installation has been completed, the PhysioQuant programme
starts automatically and the required settings can be selected.
14 / 48
3.2 Settings
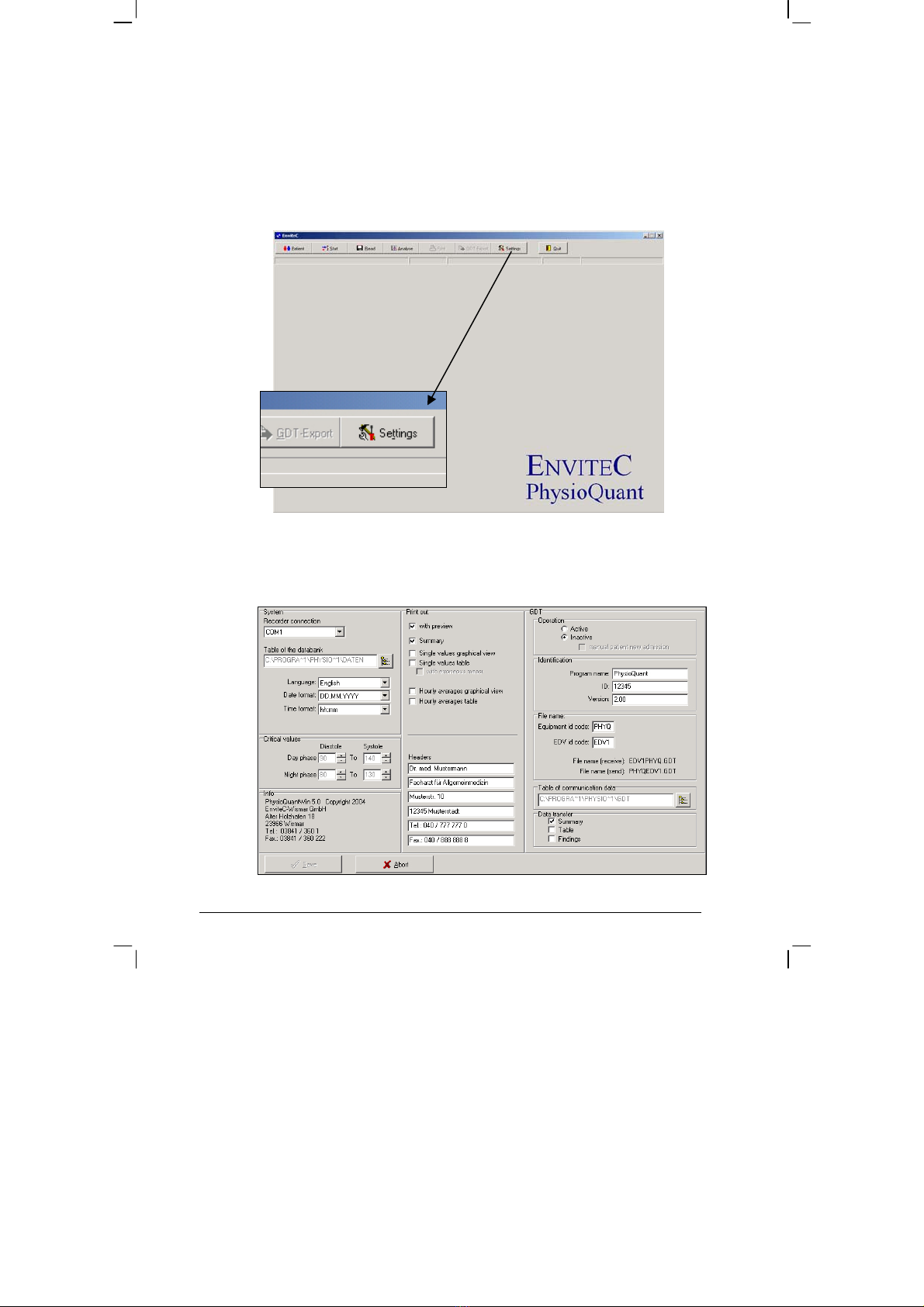
After the programme has started, the main screen will be displayed.
To configure the software, click on the 'Settings' icon to open the Settings
menu:
Settings menu:
15 / 48
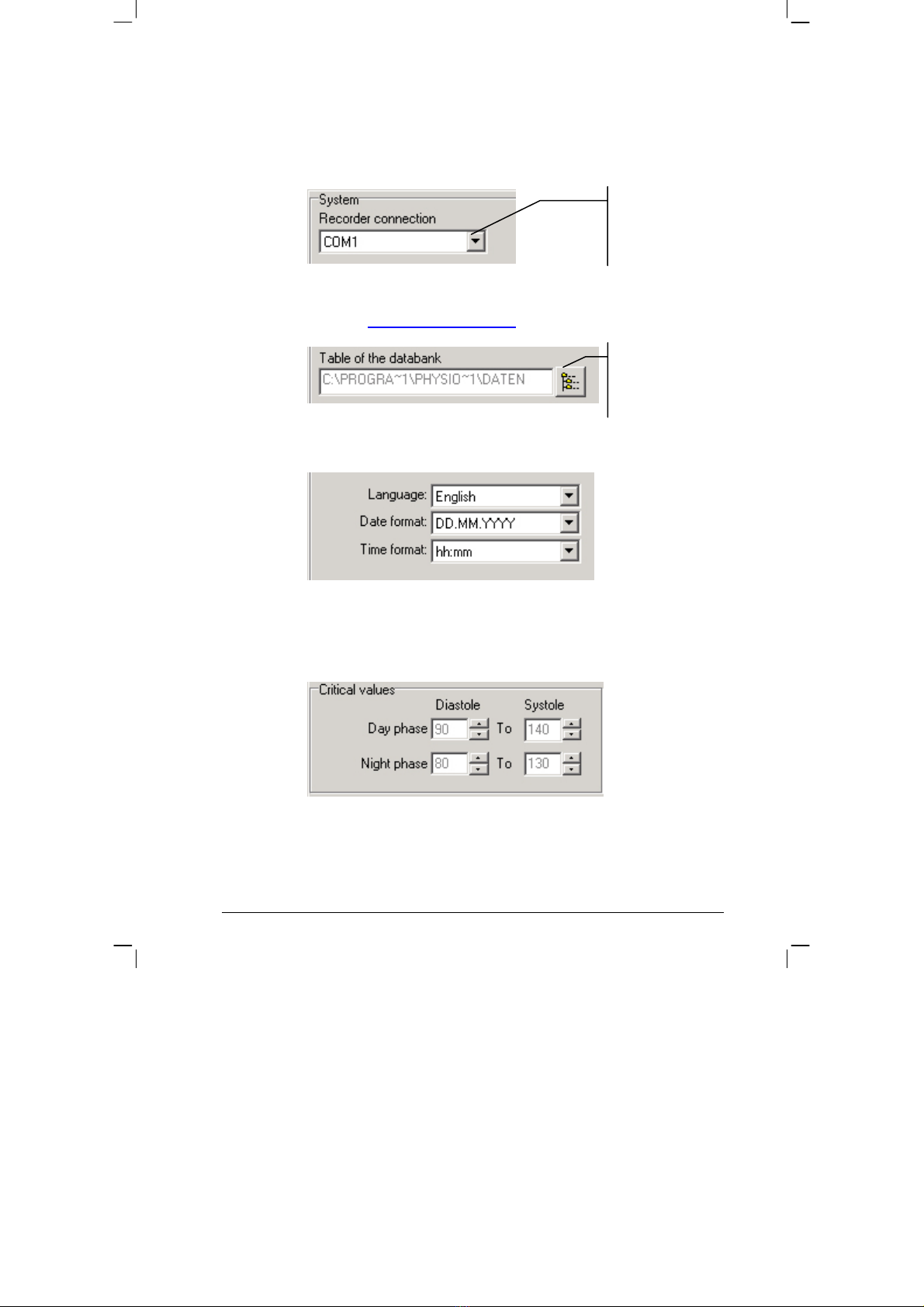
SYSTEM:
•Setting the serial PC interface, to which the PhysioQuant Recorder
will be connected:
•Setting / changing the folder for the database
(also see 3.4 Network installation, page 18)
•Setting language, date and time format:
CRITICAL VALUES:
•Setting the critical values for day phase and night phase:
•These critical values will be displayed as lines in the graphic
representation of the analysis and will be part of the statistical
calculations.
Selecting another
folder for the
database.
This displays all
serial interfaces of
the system.
16 / 48
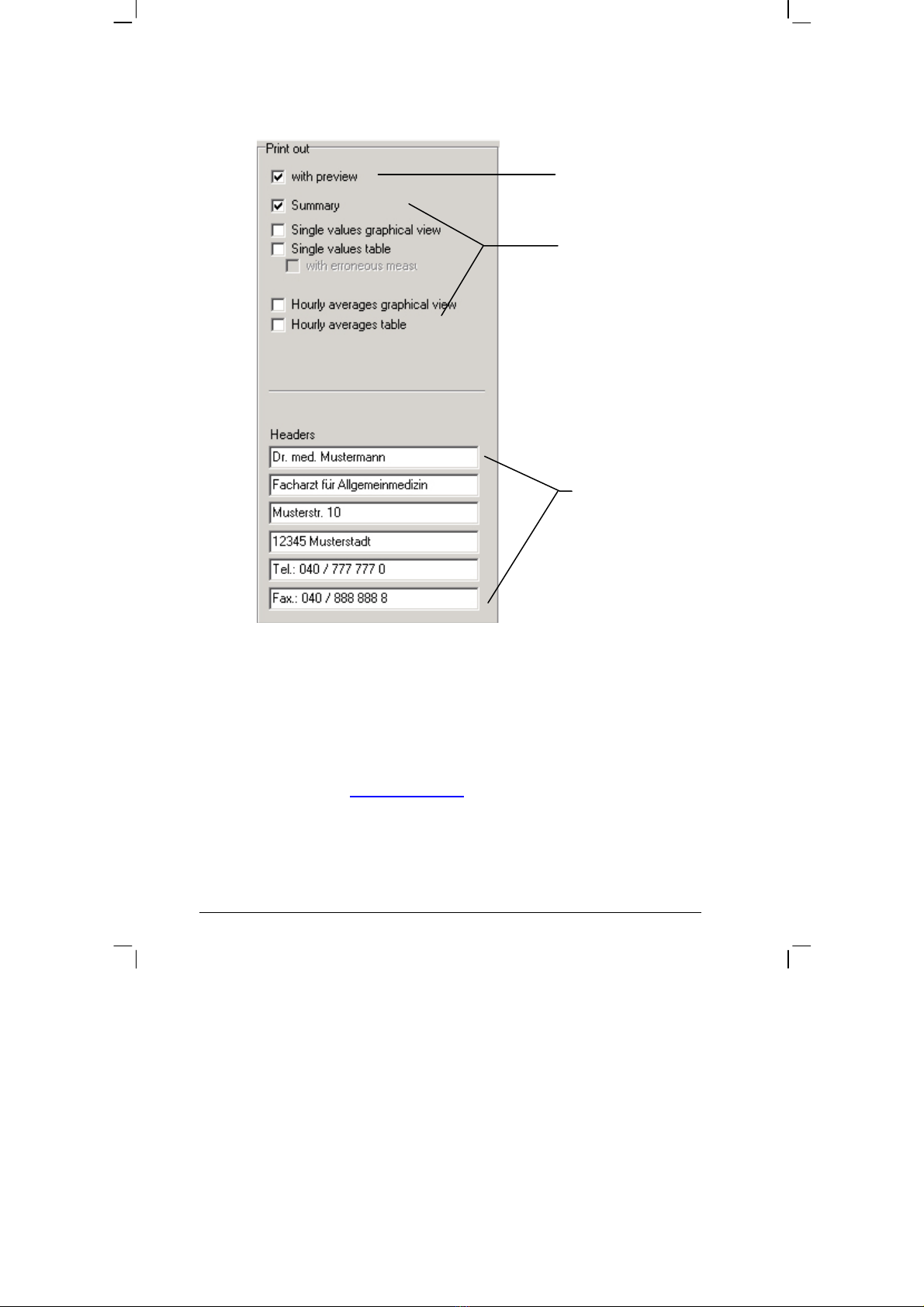
PRINT:
GDT:
The description of the GDT interface and the required settings can be
found in Section 3.5 GDT interface,18.
Activate print
preview.
Selecting standard
print pages.
Headings that
should be included
with every print-
out.

17 / 48
3.3 Attaching the connecting cable to the recorder
In order to start the recorder and read information from it, the recorder
must be connected to the PC via a special connecting cable.
This special connecting cable is connected to a free serial interface of
the PC ('COM x') via the 9-pin DSB port.
If the recorder only has a USB connection, then the connection can also
be made by using one of the commercially available USB-to-serial
converters.
Please contact your specialist dealer for more information.
The cable also needs to be plugged into the 6-pin, black port, which is
located at the back of the casing of the device. Please ensure that the
plug is in the right position when connecting it ('nose' up):

18 / 48
3.4 Network installation
The PhysioQuantWin software can be used in networks. This allows for
central storage of patient information and measurements (i.e. on the
server). This information can then be accessed from all workstations.
To this end, a corresponding directory is first created on the central
server. This folder must be connected as a 'drive' on all workstations.
A local programme installation is then carried out on all workstations that
will use the PhysioQuant software (as described in Section 3 Installation,
page 11).
The folder for the shared database is then created under [Settings],
whereby selection is facilitated by the integrated Explorer functionality:
3.5 GDT interface
The GDT interface is a standard produced by the German Quality
Assurance Medical Software (QMS – 'Qualitätsring Medizinische
Software') for system-independent data transfer between medical
devices and general practice IT systems.
The PhysioQuantWin software has an integrated GDT interface ('Device
Data Carrier interface' – 'Geräte-Daten-Träger') and therefore allows for
easy data transfer with a general practice IT system.
Please contact your general practice IT dealer to find out what the
correct settings of the GDT interface are in relation to the relevant IT
system.
All standard settings in the GDT standard (Version 2.0) can be adjusted
on an individual basis.

19 / 48
Operation
This setting activates and deactivates the GDT interface.
When the GDT interface is activated, the software will check during the
start of the programme whether the defined GDT data is available and
processes it. If no data is found, then a 'normal' programme start will take
place.
Note: During GDT operation, the option for manually adding new
patients should not be active, so as to avoid any incorrect
input or non-corresponding patient master data (general
practice IT system and PhysioQuant database).
Identification
The ID is a unique identifier that consists of a minimum of 1 and a
maximum of 8 characters, which uniquely identifies the PhysioQuant
system during GDT data transfer.

20 / 48
File names
The file names that are used for the communication between general
practice IT systems and PhysioQuant software should be entered in the
'File names' field.
File names consists of a device identification code (1 - 4 characters),
e.g. PHYQ, and a EDP identification code (1 - 4 characters) for the
general practice IT systems, e.g. EDP1.
Both these identification codes are then used to create file names, which
will always have the extension *.GDT.
Directory for communication data files
The data transfer folder can be created where you want. In order to avoid
any networks errors, a separate folder should be created for each
individual work station (e.g. on a local PC).
NB: GDT data files will need to be read and then
deleted by the general practice IT system, before
any further GDT data files can be generated.
Data transfer
Prior selection will determine which data is transferred to general practice
IT system.
Example Short summary (one line)
LZBDM: Day 108/ 67/ 78 - Night 0/ 0/ 78 = -100/-100/-100 %
Example Table
24 h BPM Day phase Night phase Difference
06:00-21:59 22:00-05:59 Day/Night phase
Mean values:
Ps[mmHg 108 0 -100%
Pd[mmHg 67 0 -100%
HR[P/min 78 0 -100%
This manual suits for next models
1
Table of contents
Other Envitec Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Weinmann
Weinmann MEDUVENT Standard Step-By-Step Instructions

Baxter Healthcare Corporation
Baxter Healthcare Corporation Colleague 3 Operator's manual
GE
GE MAC 1200 Operator's manual

Woodpecker
Woodpecker Fi-G instruction manual

VIDAR
VIDAR AdvantagePRO Series installation guide
Siemens
Siemens ACUSON Freestyle user manual