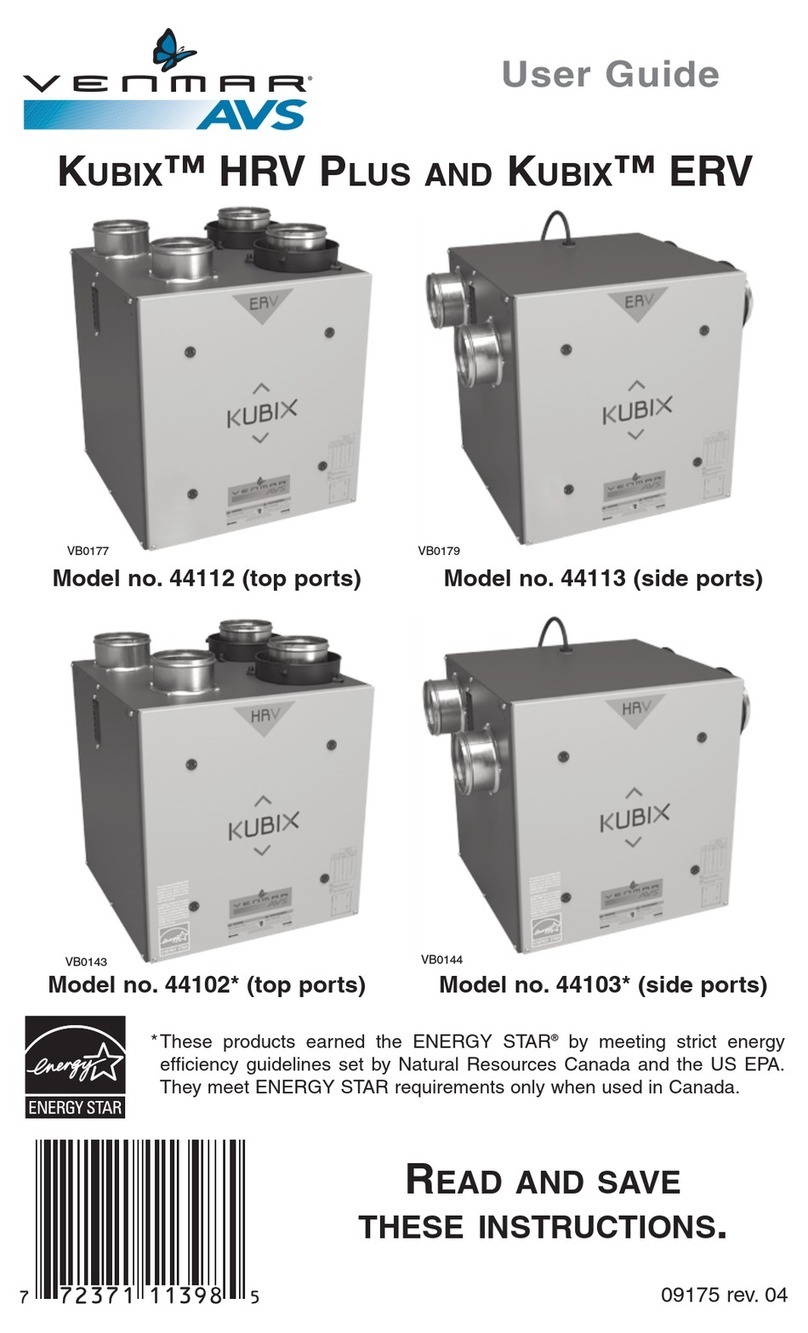
Instructions for Use: GEV019-100
Page 3
Table of Contents
1Introduction........................................................................................................................................................................ 5
2Labels ................................................................................................................................................................................. 6
3Safety Instructions.............................................................................................................................................................. 8
3.1 Safety Symbols .................................................................................................................................................................... 8
3.2 Warnings and Contraindications......................................................................................................................................... 8
4System Overview.............................................................................................................................................................. 11
4.1 Operating Concept ............................................................................................................................................................ 11
4.2 Device Description............................................................................................................................................................. 13
4.3 Single Use Components..................................................................................................................................................... 15
4.4 Patient Breathing Circuit ................................................................................................................................................... 16
4.5 Power Supply..................................................................................................................................................................... 16
4.6 Gas Connections................................................................................................................................................................ 17
5User Interface................................................................................................................................................................... 19
5.1 Gas Control Knobs ............................................................................................................................................................. 19
5.2 Human Machine Interface (HMI) ...................................................................................................................................... 20
6Operating Instructions...................................................................................................................................................... 27
6.1 Installation ........................................................................................................................................................................ 27
6.2 Pre-Start Checks and Instructions ..................................................................................................................................... 29
6.3 Default Settings................................................................................................................................................................. 29
6.4 Quick Start......................................................................................................................................................................... 30
6.5 Start Up ............................................................................................................................................................................. 30
6.6 Volume Controlled Ventilation (VCV) ................................................................................................................................ 32
6.7 Pressure Controlled Ventilation (PCV)............................................................................................................................... 34
6.8 Respiratory Rate and I:E Ratio Setting .............................................................................................................................. 35
6.9 FiO2Mixture Setting .......................................................................................................................................................... 36
6.10 Gas Flowrate Control (Slope) ............................................................................................................................................ 36
6.11 Suctioning.......................................................................................................................................................................... 37
6.12 Continuous Positive Airway Pressure (CPAP) .................................................................................................................... 38
6.13 PEEP Setting ...................................................................................................................................................................... 38
6.14 Alarms and Faults ............................................................................................................................................................. 39
6.15 Stopping Ventilation.......................................................................................................................................................... 42
6.16 Stand-by / Off-State Mode................................................................................................................................................ 42
6.17 Shutdown .......................................................................................................................................................................... 42
6.18 Unscheduled Stops ............................................................................................................................................................ 43
6.19 Battery Life Indicator......................................................................................................................................................... 44
6.20 Connecting Other Devices ................................................................................................................................................. 45
7Storage and Handling........................................................................................................................................................ 47
7.1 Packaging.......................................................................................................................................................................... 47
7.2 Short Term Storage ........................................................................................................................................................... 47
7.3 Long Term Storage ............................................................................................................................................................ 48
7.4 End of Product Life ............................................................................................................................................................ 48
8Cleaning and Maintenance ............................................................................................................................................... 49
8.1 Cleaning Procedure ........................................................................................................................................................... 49
8.2 Cleaning Agents and Disinfectants.................................................................................................................................... 50
8.3 Maintenance Schedule...................................................................................................................................................... 50
8.4 Maintenance Procedures .................................................................................................................................................. 51
8.5 Calibration......................................................................................................................................................................... 52
9Troubleshooting ............................................................................................................................................................... 53
10 Specifications and Performance........................................................................................................................................ 55