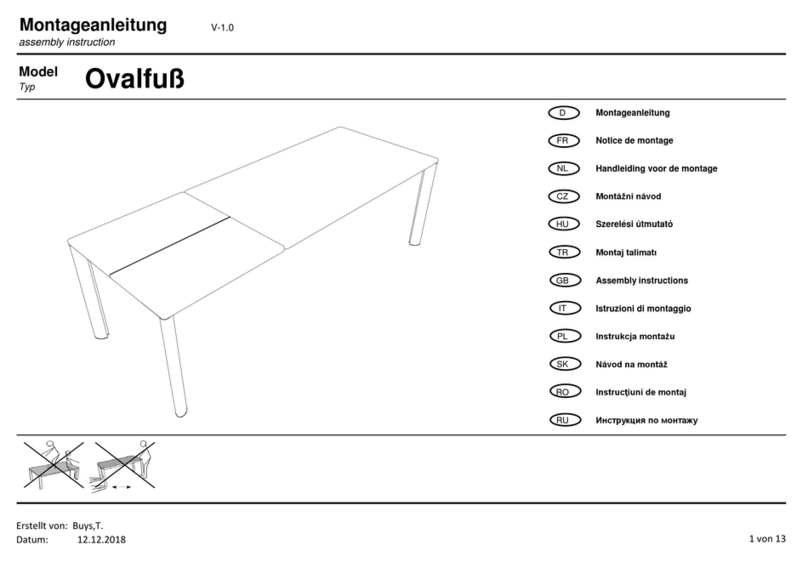
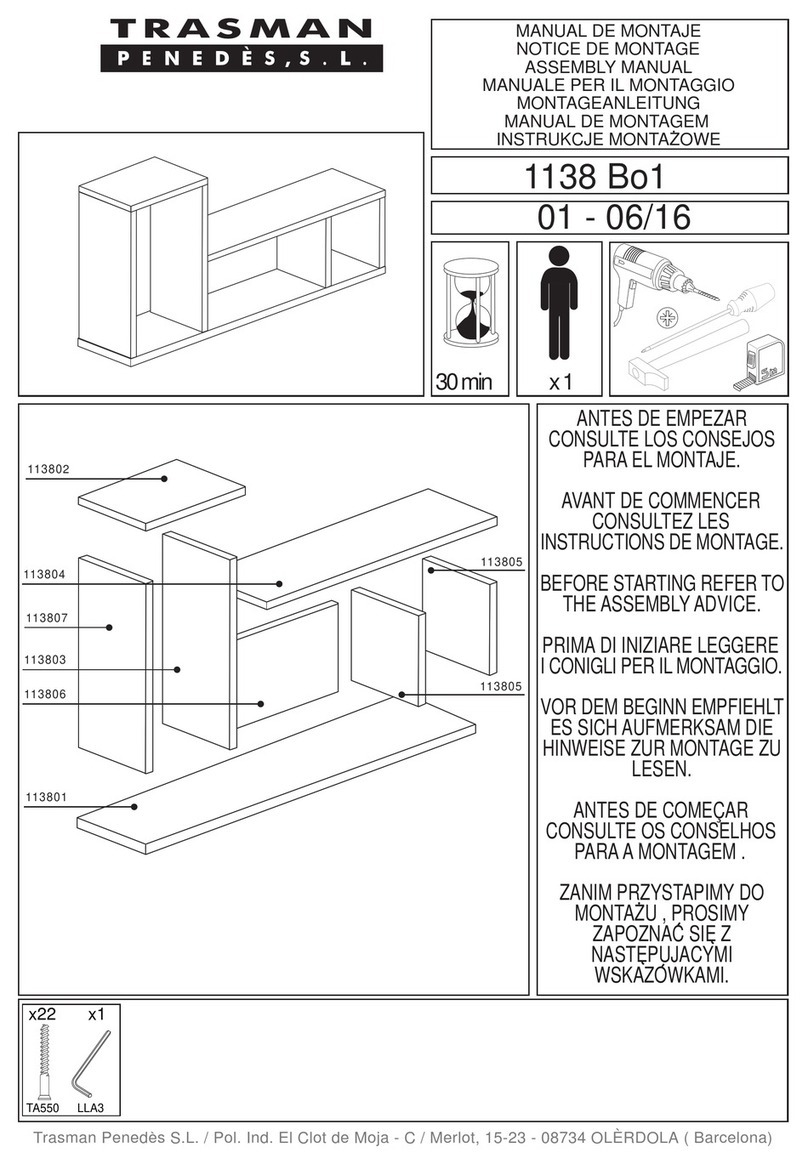
T+44 (0)1709 377172 F+44 (0)1709 377173
4
WARNINGS &
CAUTIONS
READ THIS INSTRUCTION MANUAL AND OBSERVE SAFETY
INSTRUCTIONS.
WARNING
This system must be properly installed and operated as directed by this user manual.
The system should be checked regularly to ensure correct operation. Loss of function
will remove all pressure relieving properties this system provides.
This system is intended for use as part of a pressure ulcer prevention program; do not
rely solely on this device to achieve the result. The medical professional is responsible
for applying best medical judgment when using this system.
In order for alternating air pressure range to be effective, avoid placing objects on
the surface that may obstruct the movement of air between the cells. For the same
reason, discourage people from sitting on the edge or on the end of the mattress
whilst it is in use.
All hoses must be free of kinks, twists and must be properly connected and positioned
so as not to cause any obstruction.
Do not position the system in a way that prevents access to the disconnection device
(mains power plug).
Ensure the mains lead or pump cannot become trapped or crushed, e.g. by raising or
lowering of bed or bed rails or any other moving object.
Check the mains lead is damage free and positioned so as not to cause an
obstruction, or injury, e.g. Strangulation or trip hazard.
Ensure that the electricity supply is of the type stated on the pump unit.
Protect your system from open ames. Ensure that the system is not used in the
presence of ammable anaesthetics.
Do not place device on or near a heat source or cover pump with bedding.
Harvest Healthcare advise against smoking whilst the system is in use, to prevent the
accidental ignition of associated items which may be ammable, such as bed linen.
•
•
•
•
•
•
•
•
•
•
•
•