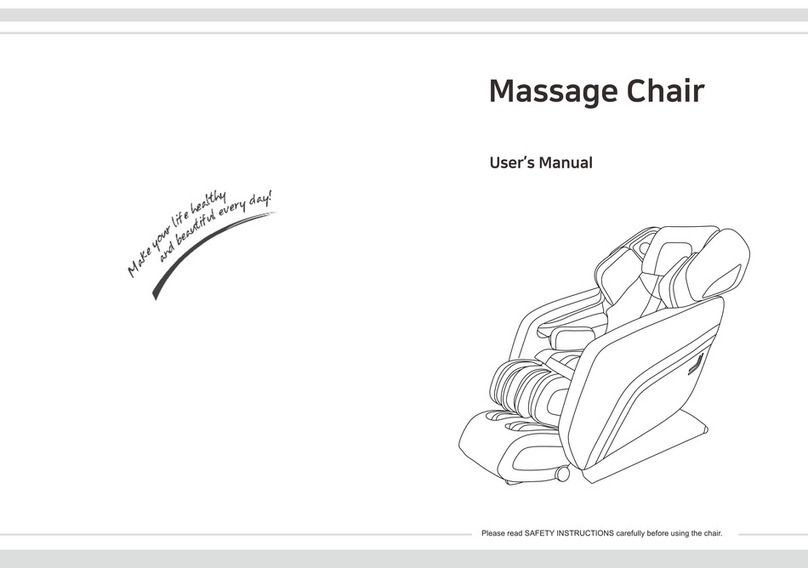
Harvest Healthcare HFC143 Installation and operating manual

Esales@harvesthealthcare.co.uk www.harvesthealthcare.co.uk 1
www.harvesthealthcare.co.uk
STATIC CUSHION
General User/ Safety Guide
HARVEST
ULTRA-THIN
CUSHION
ACTIVE CUSHIONS
www.harvesthealthcare.co.uk

E[email protected] www.harvesthealthcare.co.uk 3
CONTENTS
WARNINGS & CAUTIONSWARNINGS & CAUTIONS
OVERVIEWOVERVIEW
GENERAL INFORMATIONGENERAL INFORMATION
GUARANTEES & WARRANTIESGUARANTEES & WARRANTIES
INSTALLATIONINSTALLATION
CLEANING & CARECLEANING & CARE
DECLARATION OF CONFORMITYDECLARATION OF CONFORMITY
4
5
6
7
7
8
10

T+44 (0)1709 377172 F+44 (0)1709 377173
4
WARNINGS &
CAUTIONS
READ THIS INSTRUCTION MANUAL CAREFULLY. PLEASE NOTE
IN PARTICULAR THAT THE SAFETY INSTRUCTIONS MUST BE
OBSERVED.
WARNING
Please ensure that this booklet is read prior to rst use.
Please refer to the information in this booklet as a guideline only.
Harvest Healthcare Ltd recommend that prior to using this product, a full risk assessment
must be completed by a qualied professional, in order to determine that the correct product is
suitable for each individual.
Do not use this product for / whilst lifting the patient. This will damage the product and could put
the patient at risk.
Ensure that this product is used the correct way up.
Facial contact with the cover may cause suffocation.
Do not allow sharp objects to prenetrate the cover material.
Do not expose this product to open ames / no smoking.
Wash at
80°C (cover only
- remove foam)
Tumble dry
on Low
Do Not
Dry Clean
Do Not Bleach
Do Not Iron CE Mark No
Smoking
PHENOLS
Do Not Use
Phenols
SYMBOLS USED
This symbol indicates general hazards. There is a danger to life and health.

E[email protected] www.harvesthealthcare.co.uk 5
OVERVIEW
The Harvest Ultra-Thin Cushion has been specically developed for use with
reclining chairs. It is designed to provide enhanced pressure distribution to existing
chair cushions, without compromising the patients posture. The cushion can be
easily expanded to include leg, lumbar and arm section.
Product Code Size Max Weight
HFC143 430(L) x 430(W) x 35(H)mm 24 Stone / 158kg
HFC143LEG 440(L) x 430(W) x 25(H)mm 24 Stone / 158kg
HFC143LUM 700(L) x 430(W) x 25(H)mm 24 Stone / 158kg
HFC143COM 3 Piece Set as Above 24 Stone / 158kg
Arm rests are available on request; Product Code: HFC143ARM

T+44 (0)1709 377172 F+44 (0)1709 377173
6
GENERAL INFORMATION
INSTRUCTIONS FOR USE
Check that the safe working load of the cushion is suitable for the intended user.
Do not exceed the maximum patient weight of the cushion (see page 5).
Ensure the Risk Level is suitable for the intended user. To help prevent pressure
damage it is important for patients to reposition themselves, or are repositioned
regularly.
Cushions are delivered in plastic covers for protection; these covers must be removed
before use. Avoid using any additional covers/padding between the patient and the
support surface, as this may affect its pressure reducing qualities.
CUSHION INSPECTION RECOMMENDATIONS
Cushions should be checked regularly to ensure they remain ‘t for purpose’, clinically
effective and pose no risk of infection to either the patient or the carer.
Harvest Healthcare Ltd recommend that a thorough inspection of both the interior
(foamcore) and exterior (cover) of the cushion is carried out weekly, or each time a
new patient is placed on the cushion. Visual checks should be carried out daily to
identify any signicant signs of damage or infection risk.
If any signs of contamination are identied on the cover, the cushion should be
withdrawn from use immediately and the cover replaced. If the foam and/or the
cover are showing signs of contamination or excessive wear the cushion should be
removed from use immediately and the entire cushion replaced.
CUSHION CLEANING AND CARE
Cushions are fully encased in a protective water resistant, multi-stretch PU cover to
prevent uid i ngress from damaging t he f oam core. The cushion c over s hould be
cleaned regularly in accordance with your organisation’s disinfection protocol.
For full cleaning procedures for our cushions see page 8.
MOVING, HANDLING AND STORAGE
Where possible, cushions should be stacked at. Store cushions in a temperate, dry
environment. Do not expose to high humidity or heat. Do not drag or pull cushions
by the cover.

E[email protected] www.harvesthealthcare.co.uk 7
GUARANTEES
& WARRANTIES
WARRANTY
FOAM INNER
All Harvest Healthcare Ltd Static Foam Cushions are covered by warranty for 3
years. Damage through incorrect use will invalidate this warranty.
CUSHION COVER
All Harvest Healthcare Ltd cushion covers are covered by warranty for a period of
12 months. Damage through incorrect use and penetration by sharp instruments will
invalidate this warranty.
GUARANTEE
Harvest Healthcare Ltd guarantees to repair or replace all goods issued to its
customers which are found to be defective whilst still under their applicable warranty
period. All warranties are subject to our standard terms and conditions of sale which
can be found on our website or by request to Harvest Healthcare Ltd.
INSTALLATION
1
2
Place the seat cushion on top of the existing chair where the patient will be
seated with the clear side facing upwords. Place the straps behind the chair
and secure using the clips - tighten straps as required.
If the Seat and Lumbar section is being installed, afx the velcro to the
underside of the seat. Afx the straps for the Seat cushion as above, and
additionally hang the Lumbar straps down the back of the chair. These should
be clipped to the lower seat straps and tightened as required - please see
below.
For the Leg section, afx the velcro to the underside of the Seat cushion.
SEAT BASE STRAP
BACKREST STRAPS

T+44 (0)1709 377172 F+44 (0)1709 377173
8
CLEANING & CARE
CUSHION COVER
During general use the cushion can be cleaned by wiping with a mild detergent
solution.
Where necessary the cushion cover can be removed for laundering or sterilisation.
Where there is staining or body uids on the cushion, wash thoroughly with soap
and water, before wiping with a sodium hypochlorite solution diluted to 1000ppm
before laundering.
Cushion covers may be laundered as follows:
1Pre wash Cold 10 minutes
2Main Wash 80°C 10 minutes
3Followed by cold rinses and extraction.
WARNING
Eye protection, gloves and protective clothing should be worn when carrying out cleaning
and disinfection procedures.
When disinfecting the system, Harvest Healthcare recommends the following guidelines
which have been developed to comply with recognised infection control procedures.
These procedures are also to be used to prevent cross infection when transferring the
system between patients.
Do not use abrasive cleaners, phenol disinfectants, solvents or
alcohol-based cleansers, e.g. Dettol, Phenicol, Hibiscrub, Clearsol,
Stericol, Hycoline, as these will damage the cover materials.
Do not iron.
Ensure the cushion is thoroughly dried before use or placing in
storage.
PHENOL-BASED SOLUTIONS SHOULD NOT BE USED AS THESE
WILL DAMAGE THE CUSHION COVER.
DISPOSAL
If contaminated, please dispose of as clinical waste.
If not contaminated dispose of as household waste at your local approved facility.
Note:
Please report any serious incident related to this device to Harvest Healthcare.
Full contact details can be found at the end of this document.

E[email protected] www.harvesthealthcare.co.uk 9
ALSO AVAILABLE
HH20 - ACTIVE SYSTEM
These one-piece alternating cushions has been developed to provide maximum patient
comfort whilst achieving optimum pressure relief, whilst seated.
The Active Seat System provides relief/pressure reduction during periods out of bed. This
inexpensive system provides relief and comfort for those at risk of developing a pressure
ulcer as well as assisting in the healing of existing problem areas.
It is compatible with all of Harvest Healthcare’s pumps.
Please note that this is a cushion only, pumps are available separately.
For more information, please contact
a member of the sales team on
01709 377 172
Our standard terms and conditions of sale can be found on our website or by
request to Harvest Healthcare Ltd.
If this device malfunctions and a serious incident occurs please contact
Harvest Healthcare Ltd or your competent authority. A member of our service
team will be happy to help you and offer advice.
Telephone: 01709 377172

T+44 (0)1709 377172 F+44 (0)1709 377173
10
DECLARARTION
OF CONFORMITY
DECLARATION OF CONFORMITY
Declaration of Conformity Annex VII EU Directive 93/42/EEC
We, as company: Harvest Healthcare Ltd
Sheaf House
Bradmarsh Way
Bradmarsh Business Park
Rotherham, S60 IBW
Product Development and Quality Manager
Rotherham, 08.04.2020
Confirm on our own behalf that the medical product:
HFC143LUM UDI: 5060517530389
Risk Class: I
SRN: TBC
complies with all applicable requirements in Appendix I of the EU directive 93/42E EC
The following compliance evaluation process was applied: Annex VII
EU legislation for this type of product covers all safety, health and environmental requirements.
Modifying this product without consultation with Harvest Healthcare Ltd will invalidate this declaration
of conformity.
This declaration of conformity is issued under the sole responsibility of Harvest Healthcare.
Notified Body: TBC
This document is valid for 4 years after the date of the signature.
Authorised Representative: TBC
SRN: TBC
HFC143COM UDI: 5060517530396
HFC143ARM UDI: 5060517532017
HFC143LEG UDI: 5060517530372
HFC143 UDI: 5060517530365
Ultra-Thin Cushion

E[email protected] www.harvesthealthcare.co.uk 11
NOTES
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................
....................................................................................................................

T+44 (0)1709 377172 F+44 (0)1709 377173
12
Sheaf House, Bradmarsh Way,
Bradmarsh Business Park,
Rotherham, S60 1BW
T +44 (0)1709 377 172
F+44 (0)1709 377 173
www.harvesthealthcare.co.uk
© Copyright Harvest Healthcare
Our Full Terms & Conditions are available by request or can be found on our Website.
Harvest Healthcare reserves the right to alter or amend this document without notice.
Serial No:
DOCUMENT REFERENCE: ULTRA-THIN CUSHION - VERSION 3 - March 2020
This manual suits for next models
8
Table of contents
Other Harvest Healthcare Massager manuals