3
In case of doubt, consult a doctor or medical professional for instructions and guidance.
•
In acute emergencies, first aid takes priority.
•
Keep the unit out of the reach of children. Medical products are not toys!
•
Treatment should be monitored if used on or near children or persons who are handicapped
•
and only have a limited capability to use this device.
Packaging materials are a deadly hazard for children and can cause suffocation. Remove
•
all packaging materials immediately and keep them away from children at all times.
Do not use the device while taking a bath or a shower.
•
Do not use this device while driving a car or another kind of vehicle or while operating
•
machinery or heavy equipment.
Never use the device in rooms where aerosols (sprays) are used or pure oxygen is being
•
administered.
Do not use with heating pad or any other electrical device.
•
Do not use with AC Power source.
•
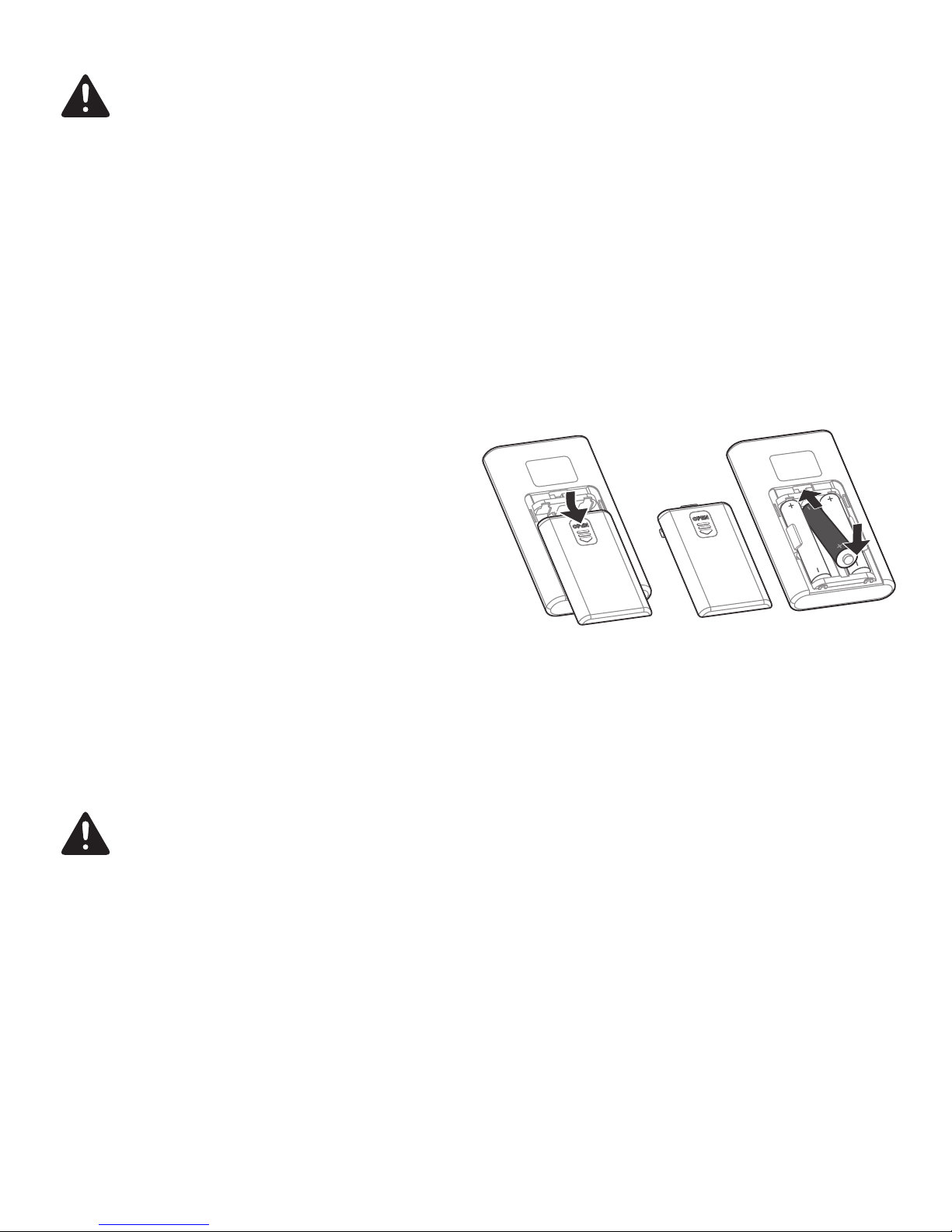
Swallowing batteries and/or battery fluid can be extremely dangerous. Keep the batteries
•
and the unit out of the reach of children and disabled persons. Should any person swal-
low a battery and/or battery fluid, please call 911 immediately.
Batteries should not be charged or reactivated by any other means. The batteries may
•
explode.
Batteries should not be taken apart, thrown in the fire or short circuited. The batteries
•
may explode.
Should battery fluid leak and come into contact with your eyes or skin immediately rinse
•
with plenty of clean water. Please call 911 immediately.
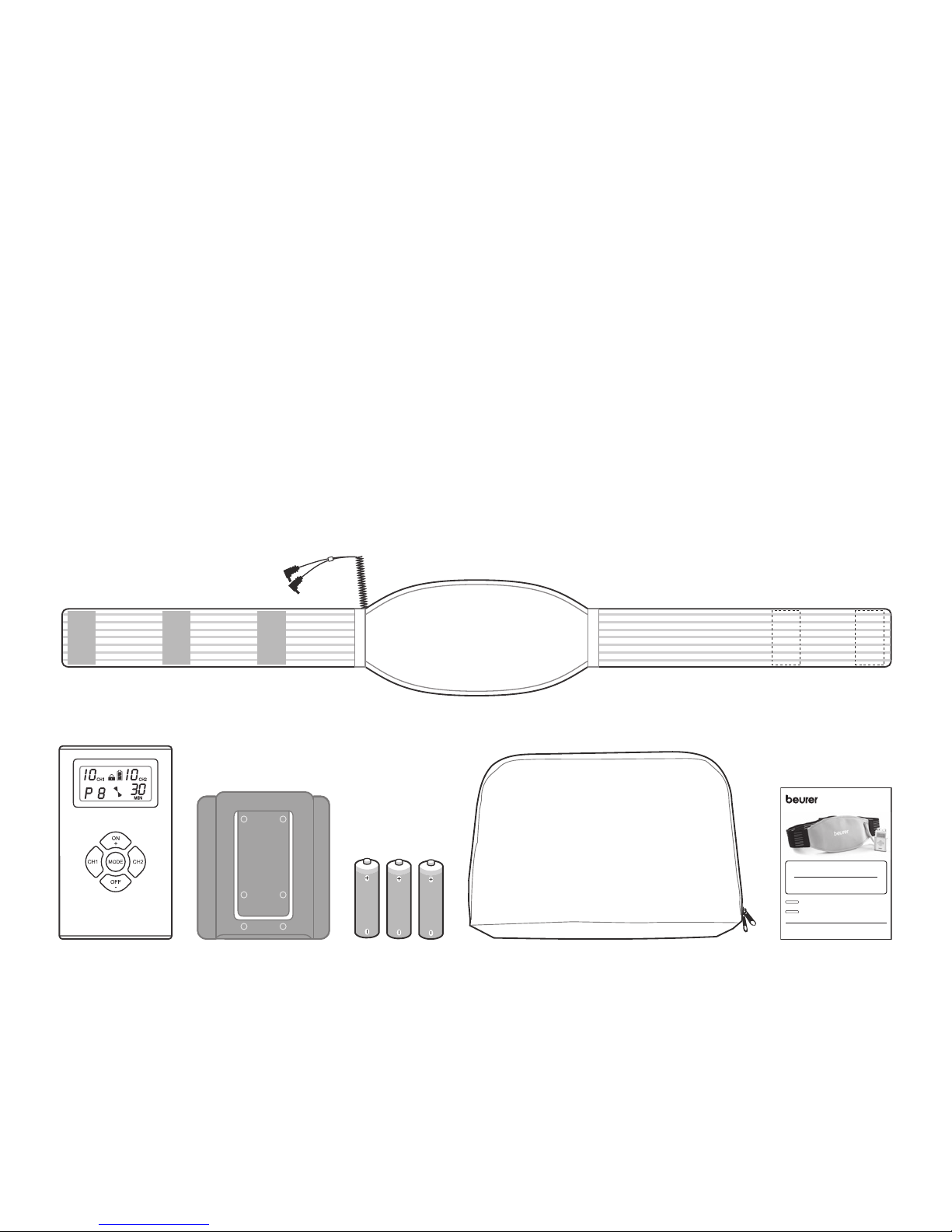
Use the unit only with the supplied accessory.
•
Do not use this unit when under influence of pain medication, alcohol or sleep aids.
•
CAUTION:
Do not use the device if it is not operating correctly, if it has been dropped, if it has fallen
•
into water or if it has been damaged.
If you feel pain or have an unpleasant feeling, stop treatment and consult your doctor.
•
Do not place the belt on:
•
– Injured or inflamed areas of skin, such as wounds, spots, rashes or reddening.
– Surgical scars in the process of healing.
– Burning or numb areas of skin.
– Swellings, peeling skin, or other sensitive points.
To prevent unpleasant electric shocks, never remove the device while it is still turned on.
•
Subjective perception of the electric current may alter with changing frequencies or pulse
•
widths. Lower the intensity as soon as the application becomes unpleasant or the pleas-
ant prickling sensation is not felt for a long period of time.
Ensure that during stimulation, no metallic objects can contact the electrodes, as other-
•
wise localized burns can occur.
Do not attempt to disassemble, open and/or repair the device yourself in the event of a
•
malfunction. You could get injured. Moreover this will invalidate the warranty.
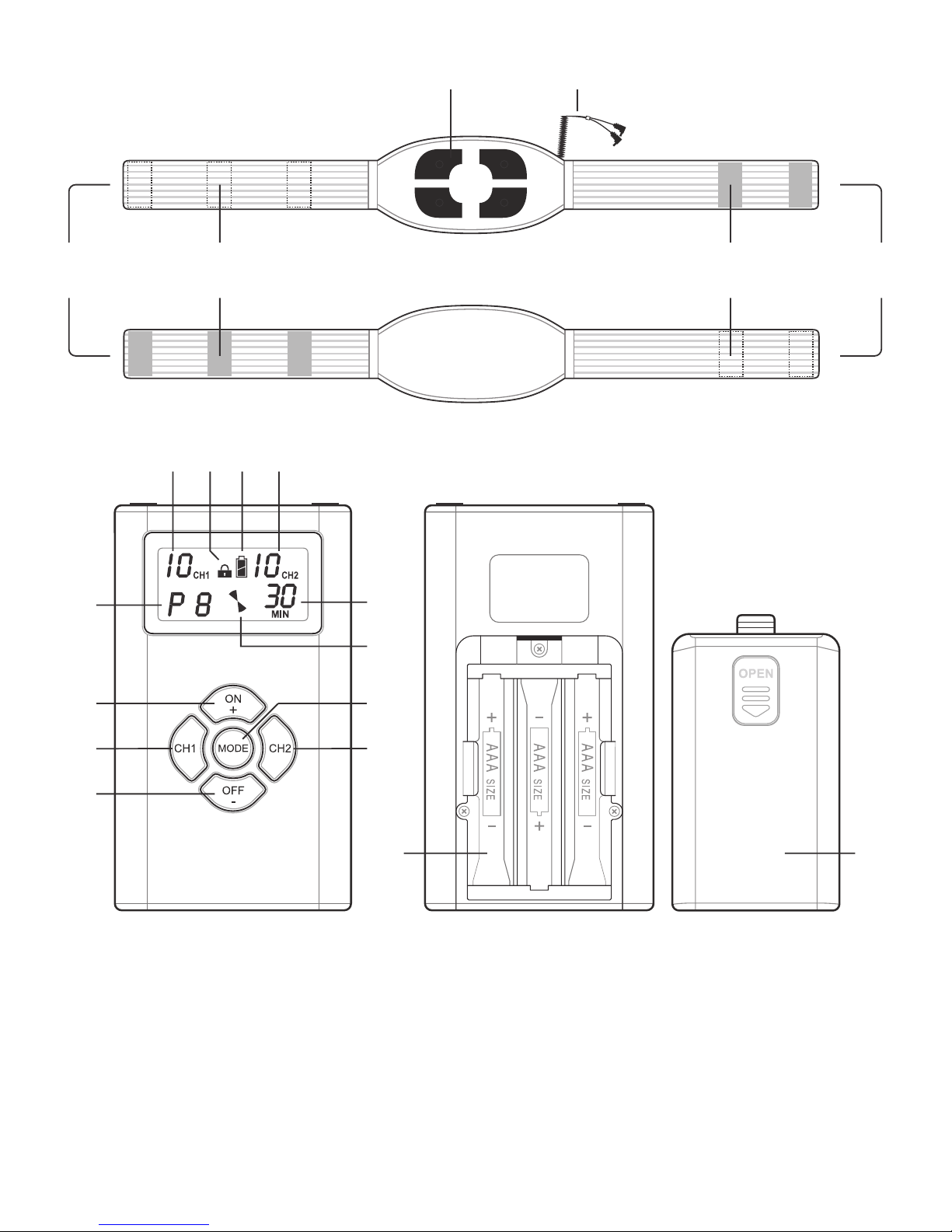
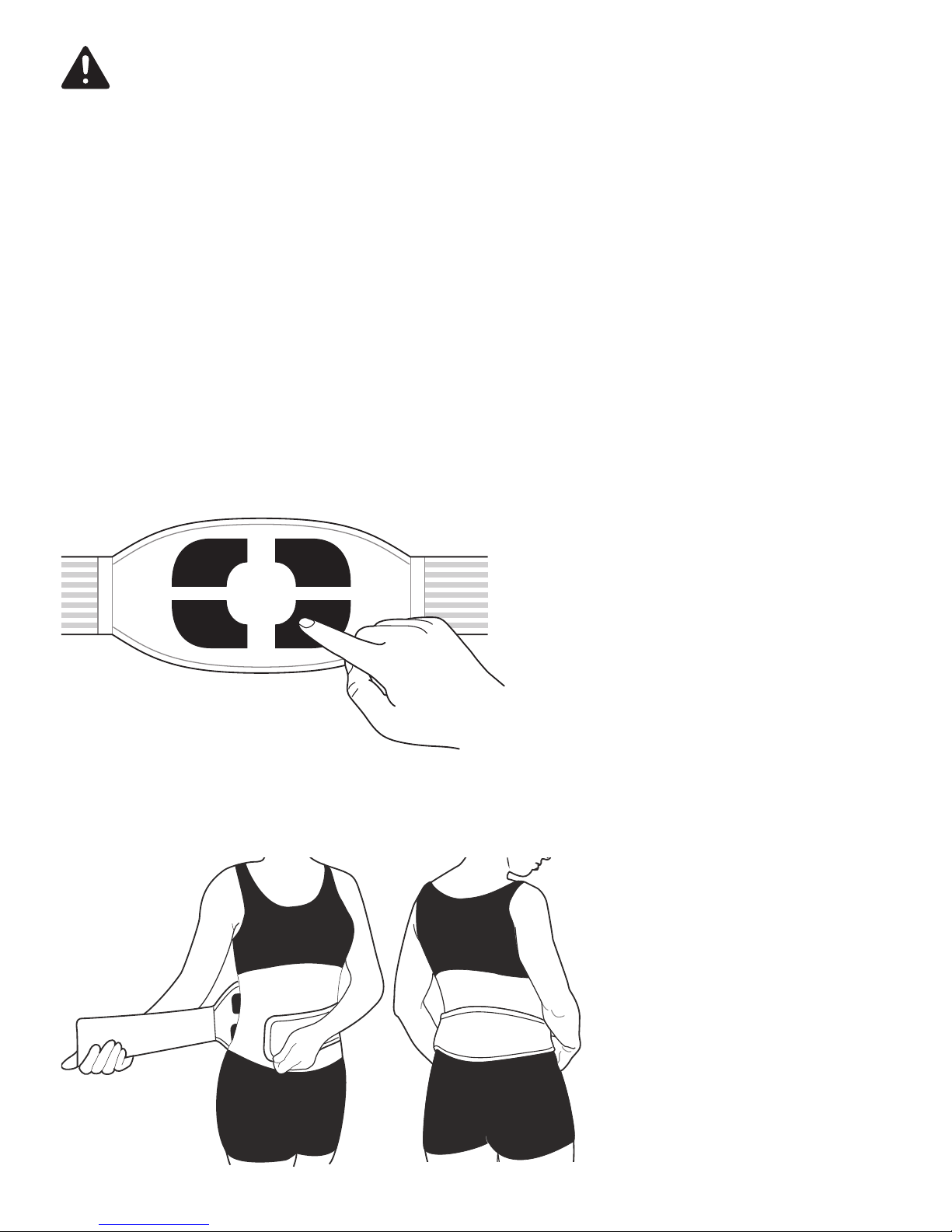
Do not switch the device on until the belt has been fixed on the body.
•
Do not use this device while sleeping.
•
Do not use with any creams, lotions or gels.
•
Do not use this device if suffering from menstrual pains.
•
Do not use the control unit and belt again until they are completely dry.
•
Do not pull on the cables but on the connectors attached to the ends of the cables in order
•
to detach the TENS control unit from the belt.