HEARTEC PRO T30 User manual

Before use, please read these instructions to help
you get the best out of your HEARTEC device.
User Instructional
Brochure
Model PRO T30
Rev 08122019.02

HEARTEC products are intended to be used only on
users over the age of 18.
HEARTEC is not intended to restore normal hearing
and will not prevent or improve a hearing
impairment.
WARNING –Choking Hazard. Keep away from
children.
-Don’ t use it at high volume for long time.
Otherwise it can injure your ear. If you feel
uncomfortable, please stop using the device.
-Batteries are harmful if swallowed. Always store
batteries out of reach of children.
-Device contain small parts which can be
swallowed. Keep hearing instruments, batteries
and accessories out of reach of children and/or
mentally disabled persons. If parts have been
swallowed consult a physician or hospital
immediately.
Important Notices
1
Rev 08122019.02

WARNING
-If parts remain in the ear, please seek medical
attention as possible, do not take it out by
yourself.
-Don’ t use the product in potential explosive or
dangerous environment.
-Don’ t use in an oxygen rich environment, and
continue use hearing amplifier for a long time.
-Do not set volume levels too high. Listening for
extended periods at high volume levels can
cause further hearing damage.
-Do not lend your hearing amplifier to other
persons. Using a hearing amplifier designed for
someone else can result in hearing damage.
Important Notices
2
Rev 08122019.02

WARNING
-Monitor any unusual skin reaction. In some
instances, wearing a hearing amplifier can cause
an allergic reaction leading to itchiness or rashes.
If you notice any unusual skin condition, stop
wearing the hearing amplifier and seek medical
attention.
-Do not touch the hearing amplifier with a
magnetic device. Hearing amplifier may be
damaged when it is touched with a magnetic
device.
-Do not use the device together with a cell
phone. Radio wave emissions from mobile
phones can cause noise in the hearing amplifier
or result in reduced volume.
Important Notices
3
Rev 08122019.02

Warning to Hearing device Dispensers
A hearing device dispenser should advise a prospective hearing device user to
consult promptly with a licensed physician (preferably an ear specialist)
before dispensing a hearing device if the hearing device dispenser determines
through inquiry, actual observation, or review of any other available
information concerning the prospective user, that the prospective user has any
of the following conditions:
(i) Visible congenital or traumatic deformity of the ear.
(ii) History of active drainage from the ear within the previous 90 days.
(iii) History of sudden or rapidly progressive hearing loss within the previous
90 days.
(iv) Acute or chronic dizziness.
(v) Unilateral hearing loss of sudden or recent onset within the previous 90
days.
(vi) Audiometric air-bone gap equal to or greater than 15 decibels at 500 hertz
(Hz), 1,000 Hz, and 2,000 Hz.
(vii) Visible evidence of significant cerumen accumulation or a foreign body in
the ear canal.
(viii) Pain or discomfort in the ear.
A hearing device will not restore normal hearing and will not prevent or
improve a hearing impairment resulting from organic conditions. In most
cases infrequent use of a hearing device does not permit a user to attain full
benefit from it. The use of a hearing device is only part of hearing habilitation
and may need to be supplemented by auditory training and instruction in
lipreading.
Important Notices
4
Rev 08122019.02

Important Notice for Prospective Hearing device Users
Good health practice requires that a person with a hearing loss have a
medical evaluation by a licensed physician (preferably a physician who
specializes in diseases of the ear) before purchasing a hearing device.
Licensed physicians who specialize in diseases of the ear are often referred to
as otolaryngologists, otologists or otorhinolaryngologists. The purpose of
medical evaluation is to assure that all medically treatable conditions that
may affect hearing are identified and treated before the hearing device is
purchased.
Following the medical evaluation, the physician will give you a written
statement that states that your hearing loss has been medically evaluated and
that you may be considered a candidate for a hearing device. The physician
will refer you to an audiologist or a hearing device dispenser, as appropriate,
for a hearing device evaluation.
The audiologist or hearing device dispenser will conduct a hearing device
evaluation to assess your ability to hear with and without a hearing device.
The hearing device evaluation will enable the audiologist or dispenser to
select and fit a hearing device to your individual needs.
If you have reservations about your ability to adapt to amplification, you
should inquire about the availability of a trial-rental or purchase-option
program. Many hearing device dispensers now offer programs that permit you
to wear a hearing device for a period of time for a nominal fee after which
you may decide if you want to purchase the hearing device.
Federal law restricts the sale of hearing devices to those individuals who have
obtained a medical evaluation from a licensed physician. Federal law permits
a fully informed adult to sign a waiver statement declining the medical
evaluation for religious or personal beliefs that preclude consultation with a
physician. The exercise of such a waiver is not in your best health interest and
its use is strongly discouraged.
Important Notices
5
Rev 08122019.02

Your PRO T30 device.....................................................7
Installing the battery.....................................................8
Operating the device.....................................................9
Placing the device into the ear..................................10
Removing the device...................................................12
Maintenance and cleaning.........................................13
Precautions for the device..........................................14
Device warranty...........................................................15
Troubleshooting...........................................................17
Te c h n i c a l data..............................................................18
Table Of Contents
6
Rev 08122019.02

Your PRO T30 Device
Rev 08122019.02 7
Microphone
Volume Switch
Mode Button
Battery
Compartment
Tubing
Ear lock
Ear dome
Part Specification Quantity
Battery #13 (1.5V) 2 Pcs.
Ear Domes Silicone 3 Pcs.
Cleaning Tools ABS 2 Pcs.
Left & Right Tubing Silicone 1 Pc. Each
List of accessories
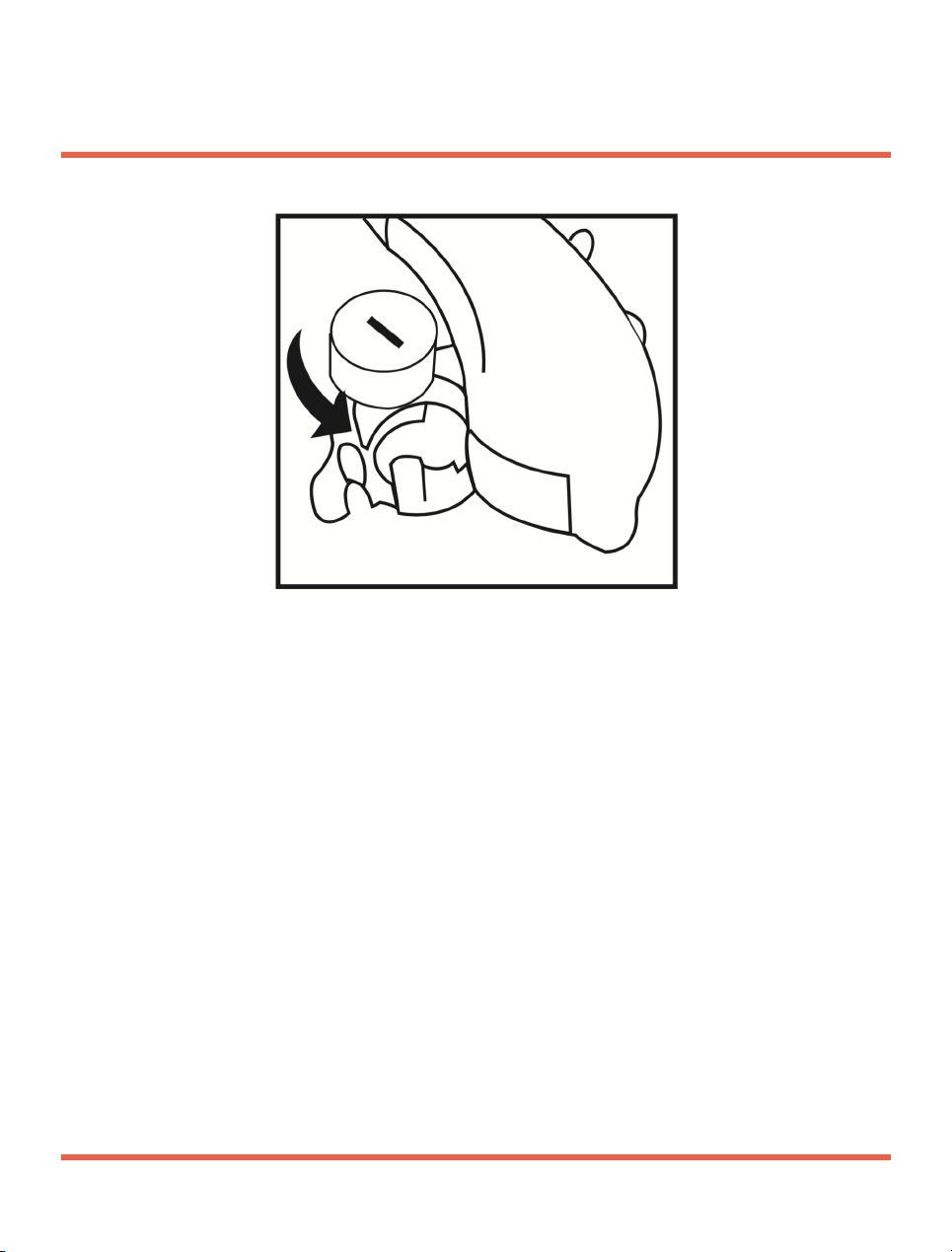
Installing The Battery
8
1. Open the battery compartment.
2. Remove the protective film from the new
battery.
3. Insert the new battery with the “+” symbol
in the correct position.
4. Gently close the battery compartment.
Rev 08122019.02

Operating The Device
9
TURNING ON and OFF
Turn ON: Close the battery compartment.
The default volume (minimum) and hearing mode
(Standard) are set.
Turn OFF: Open the battery compartment.
MODE SELECTOR
Press the “M” (Mode) button to select from the
following two modes:
1. Standard Mode
2. Noise Reduction Mode
VOLUME
Press the volume button UP or DOWN. A total of 6
volume degrees are available.
Rev 08122019.02
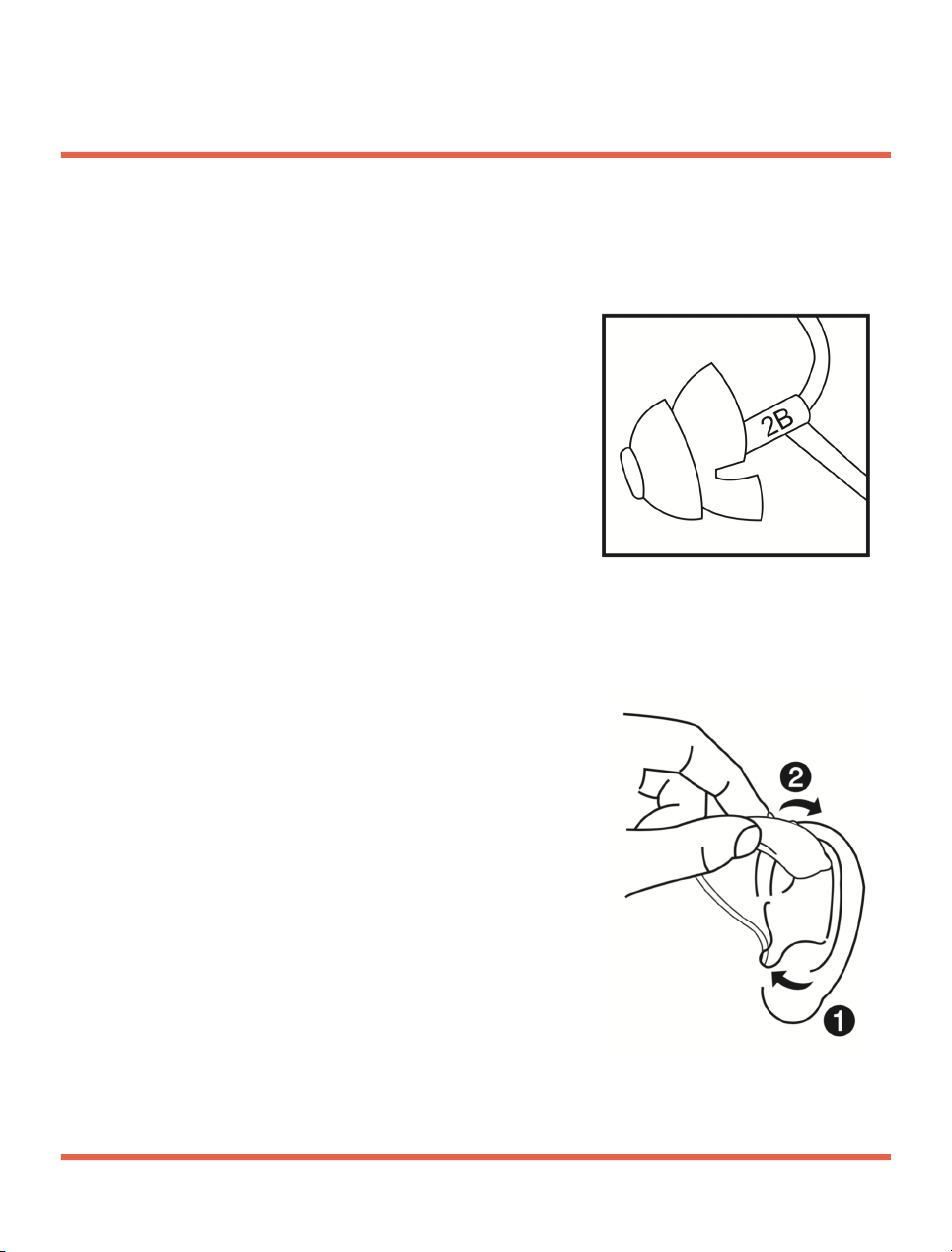
1. Please refer to the below indicator for fitting
into your right or left ear.
-marker “2B” in red = right ear
-marker “2B” in blue = left ear
2. Hold the tube at the bend that is closer to the
ear piece.
3. Carefully push the ear piece
in the ear canal.
Note: Insert the ear piece carefully
and not too deep into the ear.
4. Tw i s t i t s l i g h t l y u n t i l i t s i t s w e l l .
Note: Open and close your mouth
to avoid accumulation of air in the
ear canal.
10
Placing The Device Into The Ear
Rev 08122019.02

5. Lift the device and slide it over the top of your
ear.
6. The lock helps to securely retain the ear piece
in your ear. To position the lock: Bend the lock
and position it carefully into the bowl of your
ear (refer to the picture).
11
Placing The Device Into The Ear
Lock
Rev 08122019.02

Removing The Device
12
1. Lift the device and slide it over the top of your
ear
2. Hold the tube and pull out the ear piece
carefully.
Rev 08122019.02

Maintenance and Cleaning
13
Cleaning
For hygiene reasons and to maintain functionality,
clean your device daily.
Clean with a soft, dry tissue and provided brush.
Regularly clean the tubes.
Note: Do not put your device and accessories in
water.
Storage
During longer periods of non-use, store your
device with closed battery compartment (and
batteries removed) in a dry environment in order
to prevent the penetration of moisture.
Rev 08122019.02

Precautions For The Device
14
The following could result in damage to your
device and could void the warranty:
1. Dropping the device from a height more than
6 inches.
2. Immersing the device in water or any other
liquid.
3. “Splashing” the device with water or any other
liquid.
4. Using the device in a high humid environment
or exposing it to high heat (such as a sauna
room). Please refer to the following operating
conditions:
Operating/Storage Temperature: 32°F –104° F
Operating/Storage Humidity: < 80% RH
Rev 08122019.02

Warranty & Repairs
15
WARRANTY PERIOD
HEARTEC offers you a 1 year limited warranty valid starting from the date
of purchase. An optional second year warranty is available prior to the
conclusion of the trial period for selected HEARTEC hearing aid.
WHAT YOUR WARRANTY POLICY COVERS
This limited warranty covers defects in material and workmanship for the
HEARTEC hearing aid, which includes the hearing devices, internal
components, charger, cord and plug within the limited warranty period.
REPAIRS UNDER WARRANTY
For valid repairs, HEARTEC pledges to secure functionality at least
equivalent to the original hearing device. At the discretion of HEARTEC,
hearing devices may be replaced by new products or products
manufactured from new or serviceable used parts or repaired using new or
refurbished replacement parts. HEARTEC offers unlimited number of
repairs during warranty period for repairs covered by warranty terms.
Rev 08122019.02

Warranty & Repairs
16
EXCLUSIONS FROM WARRANTY REPAIRS
Devices purchased from unauthorized distributors (such as eBay) are not
covered by this or any other HEARTEC warranty. Damage from improper
handling or care, exposure to chemicals, immersion in water or under
stress are not covered by this or any other HEARTEC warranty. Damage
caused by third parties or non-authorized service centers are excluded
from this repair policy are not covered by this or any other HEARTEC
warranty.
HOW TO SUBMIT A REPAIR
To s u b mi t a r e p a i r r e q u e s t c o ve r e d u n d e r w a r r a n t y, p l e a s e go to the
following website:
repairs.heartecusa.com
Rev 08122019.02
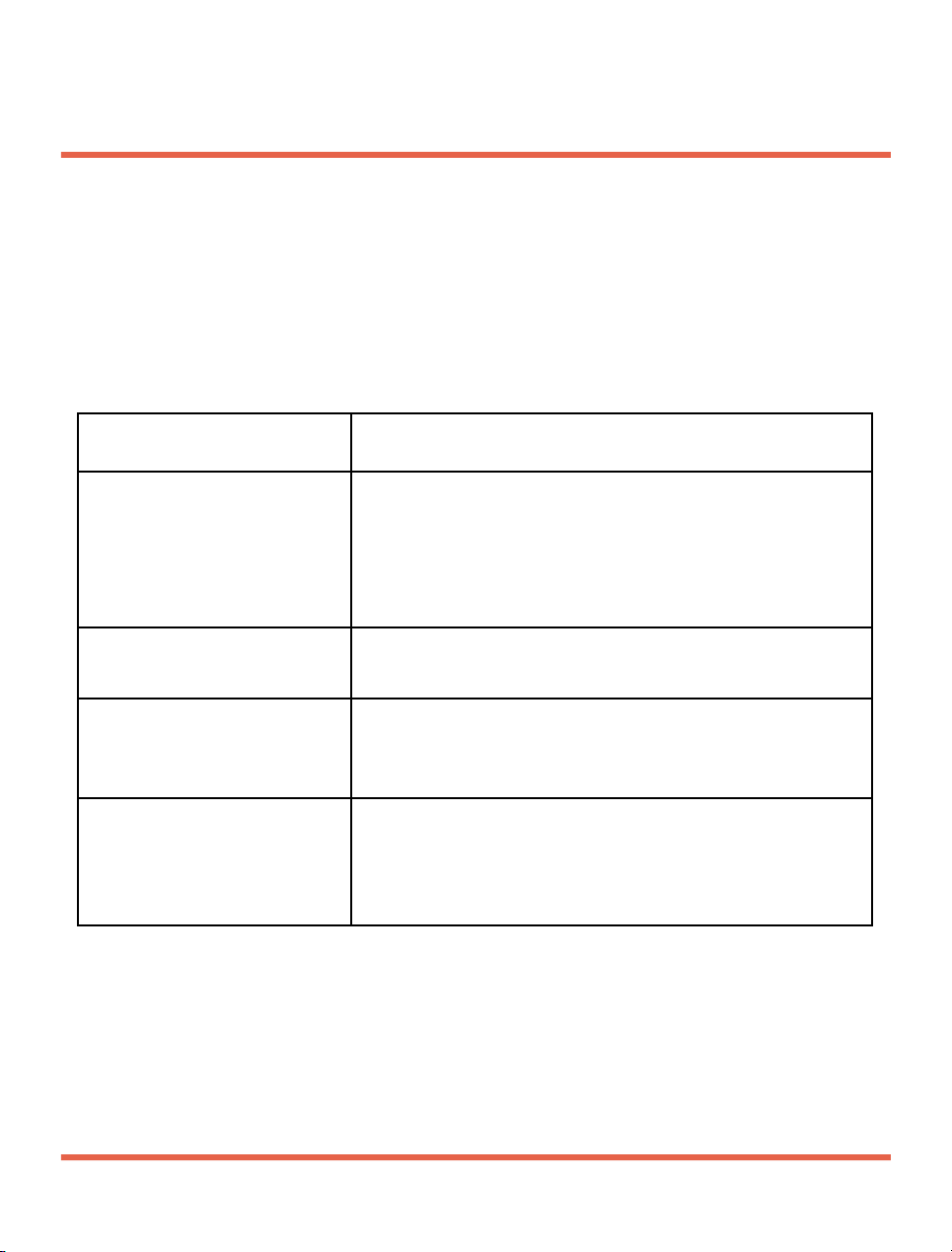
Troubleshooting
17
Check the following if the device fails during
operation:
Problem Solution
No sound or weak
sound
-
Turn on t he d e v i c e ;
-
Remove and re-install the battery;
-
Replace the battery;
-
Clean or replace the tube or ear dome;
-
Contact us.
Device emit an alert
signal
-
Replace the battery.
Sound is distorted
-
Replace the battery;
-
Decrease the volume level;
-
Clean or replace the tube or ear dome.
Device does not work
-
Turn on t he d e v i c e ;
-
Make sure the battery compartment are
closed well;
-
Replace the battery.
Rev 08122019.02

Technical Data
18
Description Specification
Ty p e Digital BTE
Power supply D.C. 1.5V , #13 battery
Max. sound pressure
level for input SSPL
90dB
122±3dB
Max. Out Put Sound
Level Gain 50±5dB
Equivalent input noise
level ≤20dB
Reference Test Gain 38±5dB
To t a l Harmonic
distortion value 500Hz≤2%; 800Hz≤2%; 1600Hz≤2%;
Frequency Range 300Hz ~ 5000Hz
Rev 08122019.02

Manufactured by:
Huizhou Jinghao Medical Technology Co., Ltd.
Distributed by:
Hearing Technologies, LLC
Rev 08122019.02
Table of contents
Popular Hearing Aid manuals by other brands

Phonak
Phonak Audeo L-RL Series user guide
oticon
oticon STREAMER PRO Operating manual and user guide

ReSound
ReSound LiNX 3D 88 quick guide

Cochlear
Cochlear Baha 6 Max Quick Guide for Android connectivity

Widex
Widex DREAM440 THE DREAM SERIES User instructions

ClearSounds
ClearSounds QUATTRO 4L quick start guide