HeraMED HeraBEAT User manual

Ultrasound Fetal Heart Rate Monitor
By HeraMED
Ultrasound Fetal Heart Rate Monitor
HeraBEAT™
The beat of life
User Manual


3
Table of Contents
1. About HeraBEATTM Fetal Heart Rate Monitor 4
2. Who should USE (Indications) or NOT USE (Contraindications) HeraBEATTM 4
3. HeraBEATTM kit includes 5
4. Safety Instructions 6
5. Ultrasound Transmission Gel 8
6. HeraBEATTM Conguration & LED Indicators 8
7. Charging HeraBEATTM 10
8. First Time Use 11
9. Measurement of the Fetal Heart Rate 12
10. Care and Maintenance 17
11. Troubleshooting 17
12. Smartphone General Security and Privacy 19
13. HeraBEATTM Specication 20
14. HeraBEATTM Device Label 21
15. Warranty and Service 22
16. Appendices 25
17. Doctor Mode 30
Read the entire user manual thoroughly prior to use.
4
About HeraBEAT™ Fetal Heart Rate Monitor Who should USE (Indications) or NOT USE
(Contraindications) HeraBEAT™
Congratulations on purchasing HeraBEATTM, your personal
Fetal Heart Rate Monitor. For the rst time ever, a safe,
eective and easy-to-use Fetal Heart Rate monitor that
delivers professional results within the comfort of your
home.
The HeraBEATTM unique software algorithm combines
ultrasound Doppler used to measure the Fetal Heart
Rate (FHR), an optical sensor to measure the Maternal
Heart Rate (MHR) and distinguish between the two to
prevent any confusion and a motion sensor used for
motion detection and control. All these enable and ensure
measurement accuracy.
HeraBEATTM will assist and guide you to locate the optimal
position for acquiring Fetal Heart Rate (FHR), measure the
Fetal Heart Rate (FHR) and store the result in a simple and
easy-to-access history le.
Intended Use
The HeraBEATTM hand-held fetal Doppler device is
intended to be self-administered and used by pregnant
women in the home environment for detecting Fetal Heart
Rate (FHR). It is an over-the-counter (OTC) device.
Indications for Use
The device is indicated for Fetal Heart Rate detection
in pregnant women starting from the 12th week into
pregnancy.
The device is indicated for normal pregnancy.
Contra-Indications
The HeraBEATTM device is NOT intended for:
Fetal Heart Rate measurements in multiple pregnancy;
Use during debrillation, electro-surgery, applying HF
surgical equipment, or magnetic resonance imaging
(MRI);.
Use during ECG measurements, on patients connected
to external electrical stimulators, or with cardiac
Pacemakers.

5
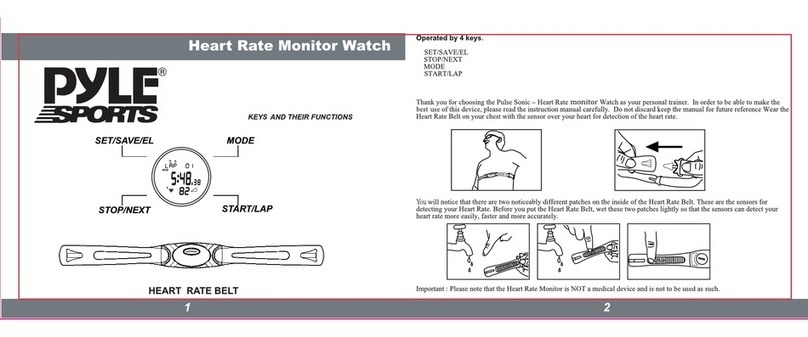
1. HeraBEATTM device
2. HeraBEATTM application (Free to download from the AppStore / Google Play.)
3. 2 Ultrasound Transmission Gel
4. Power Supply
5. Carry Case
6. User Manual
(g. 1)
HeraBEATTM device
(g. 2)
HeraBEATTM application
(g. 3)
2 Ultrasound
Transmission Gel
Check that the box includes all items. Do not use the device if it is damaged or an item is missing.
(g. 4)
Power Supply
(g. 5)
Carry Case
(g. 6)
User Manual
HeraBEAT™ kit includes
6
Always adhere to all warnings and cautions.
As with any electrical device, you must observe certain
precautions to ensure your safety
Warnings
Warnings are directions which if not followed could cause fatal
or severe injury to a user, patient or other person, or could lead
to clinical misdiagnosis, and/or loss/damage of patient-related
data.
Use this device for its intended purpose only, as described in
this user manual.
HeraBEATTM measures the Fetal Heart Rate.
For interpretation of measurement results, please consult
your physician.
The HeraBEATTM device is for personal use.
Do not use the HeraBEATTM device on or near the eyes.
Device must be charged only with the supplied power supply.
(g. 4)
Do not charge the device under direct sun light.
Use the device and its accessories only on intact skin.
Do not leave the HeraBEATTM device unattended. Store the
device and its accessories in secured place to prevent access
and use of the device by unintended individuals and
especially by children.
Do not allow water or any liquids to enter the HeraBEATTM
device or its charging connector. Do not use/store the device
near any sources of water or liquids. Do not use the device if
any liquids entered it or its charging connector.
This device is not re-resistant and cannot be used after
exposure to re or high temperatures.
Although there are no conrmed biological eects on
patients caused by exposures to ultrasound, the possibility
exists that such biological eects may be identied in the
future. Thus, use ultrasound in a prudent manner to provide
you with a medical benet.
Do not drop the device. Do not use if cracked or broken. Do
not touch the inner parts of the device.
You must check that the device is intact before use.
Discontinue use of the device with any sign of damage or loss
of cover integrity.
Should the device require service, it must be serviced only by
authorized and trained personnel by HeraMED Ltd. to
maintain safety, and reliability.
Do not use the device close to explosive or highly ammable
materials like alcohol, methanol, acetone, etc. Do not clean
the device with alcoholic materials.
Use device only with the supplied gel (g. 3). Using ultrasound
transmission gel that is not approved by HeraMED may reduce
signal quality and can lead to absence of Fetal Heart Rate
(FHR) measurement or inaccurate measurement results
.
The Ultrasound Transmission Gel is for external use only.
(g. 3) gel can be used on intact, unbroken skin.
Prior to use, verify that the Ultrasound Transmission Gel
(g. 3) has not exceeded its expiry date (located on the tube).
Gel is valid for 5 years from month of manufacture.
For any Doppler examination, it is essential that an adequate
supply of Ultrasound Transmission Gel is used to transmit
the ultrasound energy from HeraBEATTM to the surface of
the skin. Re-apply more gel if it starts to dry out or spread so
thinly that an air gap occurs between the skin and the
bottom of the device. Applying too much gel may impede the
Maternal Heart Rate measurement.
Incorrect placement of the device on the abdomen can lead
to inaccurate measurement results.
Follow the on-screen and voice instructions to locate the
optimal position for Fetal Heart Rate (FHR) measurement.
Safety Instructions
7
Do not operate this device in temperatures lower than +5°C
(41°F) or higher than +40°C (104°F), nor where humidity is
lower than 5% or higher than 95% (non-condensing), or the
device may fail or produce inaccurate measurement results.
Long term storage outside of acceptable conditions may
permanently reduce the battery capacity or lead to failure of
the device and inaccurate measurement results.
Wash hands before the measurement procedure to avoid
contamination.
Do not use the device if you are under 18 years of age.
If you have any doubt about the fetal health status after
using this device, please seek medical advice.
Do not use the device in any case that your smartphone is
damaged, or the app does not function properly.
Cautions
Cautions are directions which if not followed could cause
damage to the equipment on which the software of the medical
device is installed and/or other equipment or goods, and/or
cause environmental pollution.
External interference can cause signicant noise and impede
the measurements. Ensure that the environment in which
the device is operated is not subject to any sources of strong
electromagnetic interference, such as radio transmitters,
cordless telephones, portable and mobile RF communications
equipment, etc. Keep far away from the interference sources
no closer than 30 cm (12 inches) to any part of the device,
including cables specied by the manufacturer.
Do not dispose the device together with household garbage.
Dispose only according to electrical device’s federal, state,
and local regulations.
Notes
Notes are intended to draw your attention and aid with the use
of this medical device.
Until pregnancy week 22 - if pulse is not found after 3
attempts of 30 min each, please go to be checked at your clinic
/ doctor.
From pregnancy week 22 onwards - if pulse is not found after
one 30 min attempt, please go to be checked at your clinic /
doctor.
For successful measurement please verify that gel is applied
and device is gently pressed against the skin and held steady
without any movement.
If the displayed Fetal Heart Rate (FHR) value becomes
briey irregular, and then returns to a regular rhythm, do not
reposition the HeraBEATTM device. The irregularity might
be caused by fetal or maternal movement.
There are several physiological conditions that may impair
the ability to locate the Fetal Heart Rate:
Detection of Fetal Heart Rate in early weeks of pregnancy
can be challenging since the size of the fetus’ heart may be
very small and might be missed.
Excessive or prolonged fetal, maternal or device
movements can degrade the ability to locate and measure
the Fetal Heart Rate (FHR). If you are experiencing
frequent fetal movements during the measurement, stop
the measurement and try again once fetal movements
have subsided.
Placenta position: In cases of frontal placenta, it may take
longer to detect the pulse and will require your patience
during the search.
Safety Instructions

8
Ultrasound Transmission Gel HeraBEAT™ Conguration & LED Indicators
Use device only with the supplied gel (g. 3). Using ultrasound
transmission gel that is not approved by HeraMED may reduce
signal quality and can lead to absence of Fetal Heart Rate
(FHR) measurement or inaccurate measurement results.
The Ultrasound Transmission Gel (g. 3) is for external use only.
Gel can be used on intact, unbroken skin and on all patients
in facilities where cross contamination is of minimal concern
.
Prior to use, verify that the Ultrasound Transmission Gel (g. 3)
has not exceeded its expiry date (located on the tube). The
gel is valid for 5 years from month of manufacture.
Contraindications
The Ultrasound Transmission Gel (g. 3) should not be used in
:
Any invasive procedures in which a device is passed
through the tissue.
Any procedure on or near broken skin, such as fresh
surgical site or an open wound.
Patients with an immunodeciency or on
immunosuppressive therapy.
Any neonates or critically ill patients.
Mucous membranes.
Critically ill patients or patients in contact-, airborne- or
droplet-transmission-based precautions.
Warning and precautions
Discard product if package is damaged or evidence of
contamination is present.
Do not use if known sensitivity to parabens exists.
To avoid contamination, tip of the container should not come
into contact with contaminating elements. Apply the gel to
HeraBEATTM surface, and not directly on your skin.
Storage conditions
Store in a cool dry place. Store away from direct sunlight.
The HeraBEATTM measures the Fetal Heart Rate, processes
the data and transmits the information wirelessly to the
HeraBEATTM smartphone application (the HeraBEATTM App)
using safe BLE communication channel. The HeraBEATTM
App receives the digital information and displays the data
to the user.
The HeraBEATTM App guides the user step by step, to
easily locate the Fetal Heart Rate (FHR), displays the Fetal
Heart Rate values, stores the Fetal Heart Rate in the
smartphone's memory, provides access to results history,
and indicates the status of battery and connection with
HeraBEATTM.
HeraBEATTM LED Indicators
The device provides you with the following status
indicators:
Device turned OFF - light is OFF
Device turned ON (waiting to be connected to the App)
– constant white light
Device is connected to the App – fast ashing of white
light
Device in measurement state – Ultrasound is ON– slow
ashing of white light
Device requires charging – device is ashing a white light
3 times and turns OFF
Device is charging – orange light ON
Device is fully charged – green light ON
Review the device lights in the following two illustrations.

9
HeraBEAT™ conguration
White light App Home
Screen
Orange / Green
charging lights
Measurements History
HeraBEATTM Measurement
Tap on the Home Screen to start
measurement session.
Tap to start Fetal Heart Rate Search
in every new position
Tap to end the Fetal Heart
Rate measurement
Bluetooth Low Energy (BLE) connection
indicates that HeraBEATM is connected
to your smartphone. (BLE connected).
indicates that HeraBEATTM is NOT
connected to your smartphone.
(BLE NOT connected).
GO
END
START
Tap on the Home Screen to access the
Measurements History.
Recorded measurements will be displayed by
pregnancy week.
App Settings Menu App Settings Menu
Tutorial
Support
About
Change Language
Advanced Settings
Measurement History
Tap on the Home Screen to access
the in-app settings menu
Tap to access the Tutorial.
Tap for Support contact details
Tap to view the App version
Tap to change the App language
Tap to access the Advanced Settings.
You can edit your Personal Information, Unpair
Device, Sign In to Doctor Mode or Reset all App
Private information.
Note!
Device Unpairing will separate the connection
between the HeraBEAT device and the app and
will allow to connect a new device to the app.
Reset will permanently erase your personal
information and all measurements history from
the App. We recommend resetting when you
want to use the device for a new pregnancy.
Instructions for Doctor Mode can be found in
section 17.
i

10
Charging HeraBEAT™
1. Use only supplied Power Supply for charging. (g. 4)
2. Remove the charging connector cover.
3. Connect the Power Supply Cord to the HeraBEATTM device
4. Plug the Power Supply into an electrical outlet.
5. During charging orange light illuminates. Once fully charged, the orange light turns green.
6. Unplug the Power Supply from the electrical outlet and then disconnect its cord from the HeraBEATTM device.
Before you turn ON and start using the device, fully charge it.
When the HeraBEATTM device lights ash 3 times and the device turns OFF, it’s time to charge it.
The device cannot be used during charging.
Do not charge the device under direct sun light.
Full charge time is approximately 4 hours.
It is recommended not to over-charge the device.
When device is not in frequent use, it is recommended to periodically charge it every 3 months.
11
First Time Use
Note: The device must be operated using the smartphone
application. Visit our website for a list of supported devices.
Make sure you fully charge your HeraBEATTM prior to rst use.
Install HeraBEATTM App on your smartphone:
Go to AppStore / Google-Play on your smartphone.
Search for ‘HeraBEATTM’ app in the AppStore/Google-Play
and install it on your smartphone.
When the App starts for the rst time, the Tutorial will guide
you how to use the device.
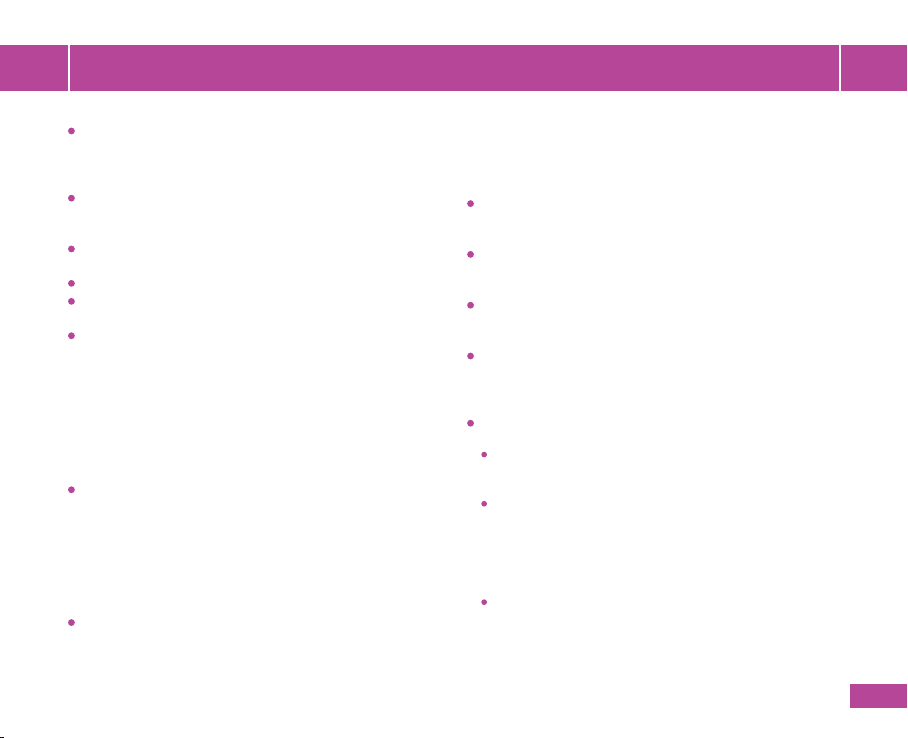
Fill in your personal information:
Last menstrual period date, Maternal date
of birth & Number of previous pregnancies.
HeraBEATTM will optimize the guided
search based on the gestational age
(12-18, 19-24, 25-42). The search will
recommend the lower abdomen area for
pregnancy weeks 12 to 18.
All measurement results are recorded
according to your pregnancy week.
Please make sure that you ll in your
personal information accurately!
Turn HeraBEATTM ON by giving it a little
shake, or by unplugging it from the power
supply (g. 4). A White light will quickly
turn on.
Note: The device will turn itself o
automatically 2 minutes after it is not in
use and disconnected from the App.
The app will automatically search for and attempt to
connect to a HeraBEATTM device.
When a device is found, the app will ask if the HeraBEATTM
device is ashing a white light for verication.
Tap ‘YES’.
The app will connect to the device. This is only for rst
use, next time the app nds the same unique
HeraBEATTM device, it will automatically connect to it.
If the connection to HeraBEATTM has not been
established, verify that the HeraBEATTM device is turned
ON (a constant white light will show), then stop the app
and re-launch it.
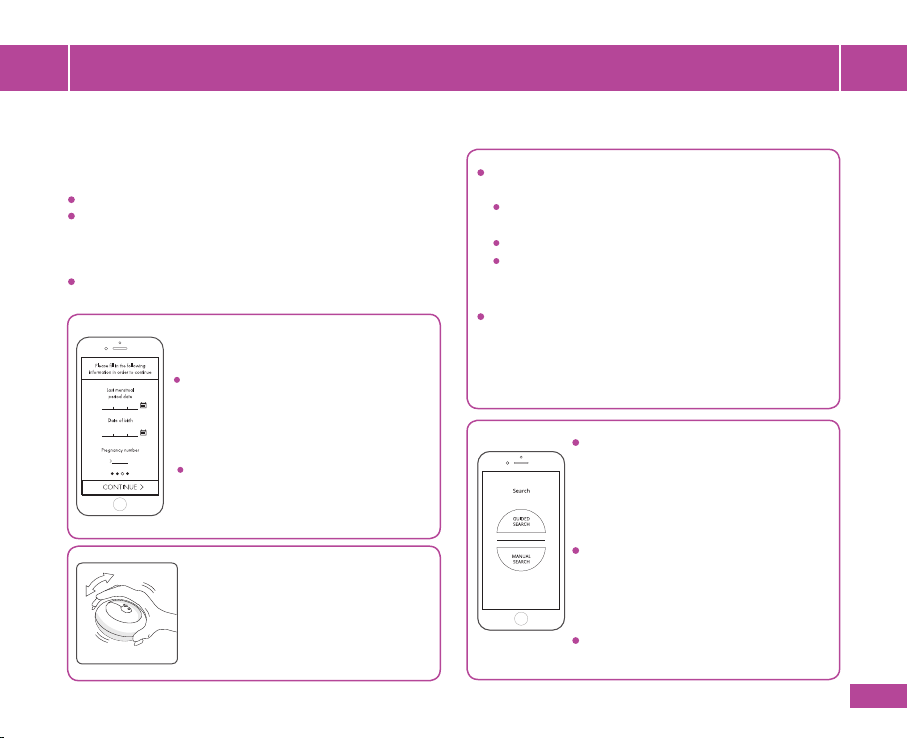
To assist you with the Fetal Heart Rate
Search, during the rst 3 measurement
sessions, the search will be guided by
the app. Starting from the 4th
measurement you can select the
Guided Search or the Manual Search.
In Guided search the app will instruct
you where to position the device on
your abdomen. In manual search, you
can select where to position the device
based on your experience.
You can return to the Tutorial anytime
from the App settings menu.

12
Measurement of the Fetal Heart Rate
1. Before starting a Measurement session
2.1 HeraBEATTM preparation
Turn the HeraBEATTM device ON by
giving it a little shake, or by unplugging
it from the power supply (g. 4).
A White light will quickly turn on.
Note: The device will turn itself o
automatically after 2 minutes if not
used and disconnected from the App.
To perform measurement with no
disturbances, it is recommended to
switch your smartphone to Airplane
Mode (Airplane Mode ON).
Otherwise, any incoming cellular
communication will stop the
measurement.
The HeraBEATTM device communicates
with your smartphone via Bluetooth
Low Energy, therefore please ensure
that your smartphone’s Bluetooth is
turned ON (Bluetooth ON).
Before you begin a search, please
make sure you have available with
you: HeraBEATTM (g. 1), your
smartphone (g. 2), the supplied
ultrasound transmission gel tube
(g. 3) and a clean paper tissue/
towel.
It is recommended that you nd a
comfortable position before starting
any measurement session. Lean back
on a pillow in bed or on a couch.
2. Measurement preparations
2.2 HeraBEATTM Smartphone preparation
13
Measurement of the Fetal Heart Rate
2.3 HeraBEATTM APP preparation
3. Applying the Ultrasound Transmission Gel:
4. Fetal Heart Rate Search
Start the HeraBEATTM App.
Wait for HeraBEATTM to connect. The App will automatically create a Bluetooth
connection which will be indicated on the screen.
Once connection with HeraBEATTM is obtained, it will rapidly ash a white light.
Please verify the device is turned ON, otherwise turn it on by giving it a little shake.
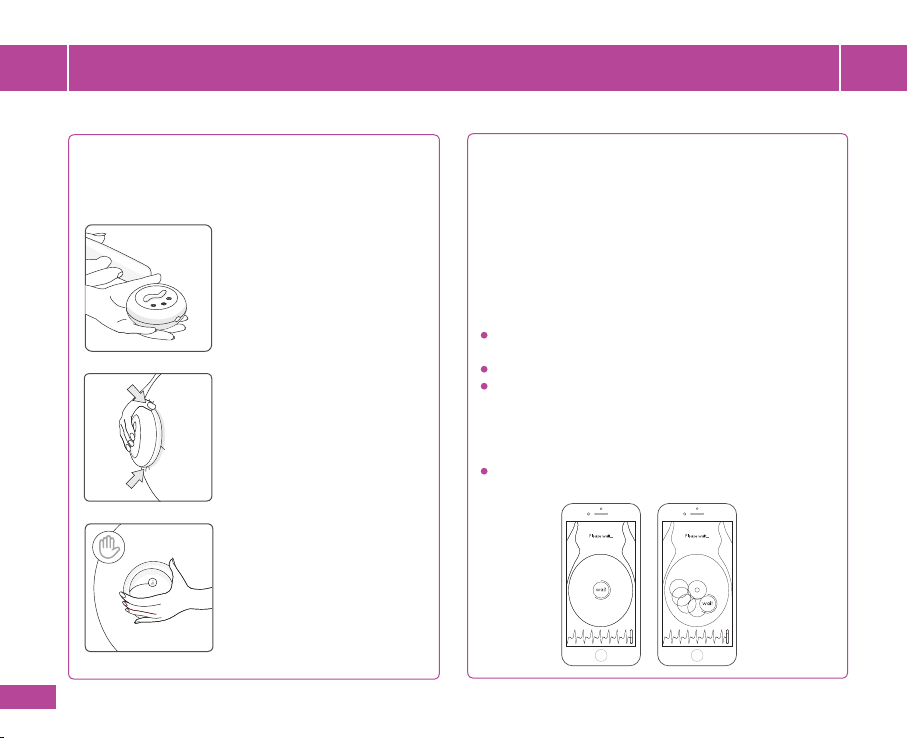
3.2
Place HeraBEATTM bottom side on your abdomen area and position over the bellybutton.
3.3
Hold the device in the palm of your hand and press it gently against your abdomen. Perform a circular motion
to ensure a thin and even layer of gel between HeraBEATTM and the skin.
3.4
Re-position the device over the bellybutton and make sure the bottom surface is fully touching your skin during
the entire measurement.
4.1
Tap the ‘START’ button.
HeraBEATTM will guide you through several search positions, while it is
searching for the Fetal Heart Rate. For each search position, tap the ‘GO’
button and wait while keeping the device steady.
HeraBEATTM will optimize the guided search based on your pregnancy week.
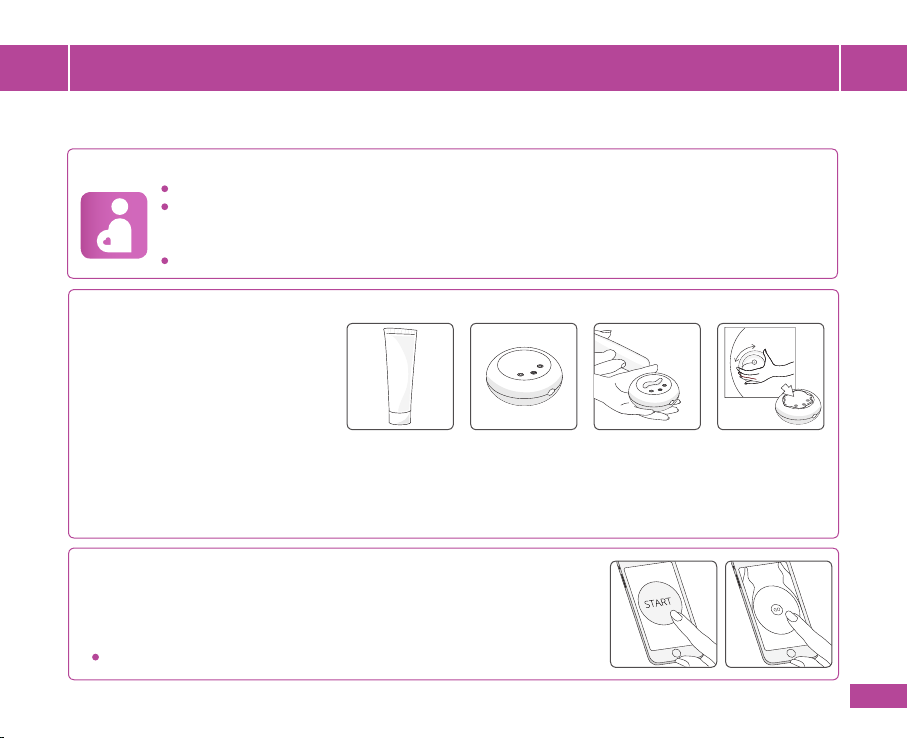
3.1 Apply a drop of supplied ultrasound
transmission gel (g. 3), about the
size of your little nger, to the bottom
surface of the HeraBEATTM device.
Bottom Surface
14
Measurement of the Fetal Heart Rate
4.2 Follow the on-screen instructions, while
maintaining the following:
3.
Hold HeraBEATTM steady while it is
searching and measuring.
1.
There is always a thin and even
layer of gel between HeraBEATTM
and your skin. Note that the gel
will dry after several minutes.
Add gel as required to maintain
a thin, moist layer.
2.
Apply a small amount of pressure
on HeraBEATTM against your skin.
Verify full contact during the
entire measurement.
4.3 During the search
Note: The measurement is sensitive to noises which occur
due to talking, eating, heavy breathing or moving. Please
refrain from these actions during the measurement.
Abdominal activity or fetal movements detected may
prolong the search duration, you may be requested to wait
in your current position to allow the device to locate the
fetal heart rate.
At the bottom of the screen a running graph will
indicate device activity.
Next to the graph, a level bar displays the signal level.
If the Fetal Heart Rate is not detected at a certain
position, you will be asked to move HeraBEATTM to
the next position (the new position will be displayed
on the screen). The distance between search positions
is about 2 ngers wide.
The app will remind you to add more gel, if
necessary, every 5 search positions.

15
Measurement of the Fetal Heart Rate
5. Fetal Heart Rate Measurement
The HeraBEATTM app will inform you when the Fetal
Heart Rate is detected. The app will then move to
the measuring screen.
Hold steady while HeraBEATTM is measuring the
Fetal Heart Rate.
The App will present and record the measurement.
The measured Fetal Heart Rate is displayed in the
center of the screen and a Fetal Heart Rate sound
is played. Results are presented in Beats Per Minute
(BPM).
The white depleting ring around the Fetal Heart
Rate value represents the duration of successful
measurement, limited to ve minutes.
The time duration for the measurement session is
displayed at the top of this screen.
Rotating the screen to landscape will display a real-
time graph of the measured Fetal Heart Rate.
If fetal heart rate tracking is lost during measurement,
the App will display ‘Wait’ instead of the fetal heart
rate value. Keep the device steady until the fetal
heart rate is located again. If the fetal heart rate is
not located again after 20 consecutive seconds, the
App will move back to the Search Screen in order to
guide you to locate the fetal heart rate.
6. END Measurement Session
Once the fetal heart rate has been recorded, the app
will oer you to end the measurement. You may also
choose to continue, in which case the measurement
will complete a 5 minute session.
You can always choose to end the measurement at any
time by tapping the ‘END’ button, or by closing the App
or by Switching to another App.
The measurement will end upon an incoming call.
You can start the measurement again once you return
to use the app. ‘Airplane Mode ON’ will allow you to
perform the measurement with no interruptions.
The HeraBEATTM device switches o automatically if no
operation is performed for 2 minutes and the device is
not connected to the smartphone.
Turn o the Airplane Mode on the smartphone.

16
Measurement of the Fetal Heart Rate
8. Measurements History
All recorded measurements are kept and organized by
pregnancy weeks in the history screen.
Tap any week to see its measurements shown below.
Tap a specic measurement to see its results screen.
7. Measurement Results
Once the measurement has nished, the measurement
results screen is shown.
The measurement results screen includes:
A test name at the top including pregnancy week,
date and time
An average Fetal Heart Rate
A graph presenting the measured Fetal Heart Rate
over time.
Measurement duration (total time from search start
to measurement end).
Rotate smartphone screen to landscape to view the
entire graph in full screen.
A dashed vertical line within the measurement results
screen indicate a loss and regain of the Fetal Hear t Rate
during the measurement.
Use the Share key to share the
measurement results. Make sure
to switch OFF airplane mode in
order to share measurements.

17
Problem: The HeraBEATTM device is not connected
or the app and device frequently disconnect.
Details: The ‘No connection’ message or icon is displayed.
Solution:
1. Verify that HeraBEATTM is located within 3 meters
from your smartphone.
2.
Ensure that Bluetooth on the smartphone is turned ON.
3. Ensure that HeraBEATTM is ON.
Turn HeraBEATTM ON by giving it a little shake, or by
plugging and unplugging it from the power
supply after charging (g. 4).
The white light ring on the top
part of the device will quickly
turn on.
Note: The device will turn
itself o automatically after
2 minutes if not in use and
disconnected from the App.
4. Close the HeraBEATTM app
and restart it.
Care and Maintenance Care and Maintenance
Troubleshooting
Warning: Never submerge the device in
liquids. Do not allow any liquid to enter the
device and especially the charging connector.
Do not use strong solvent, for example,
acetone. Never use an abrasive such as steel
wool or metal polish.
The device is not intended to be sterilized.
Cleaning
Before cleaning HeraBEATTM , ensure that it is
unplugged from the power supply (g. 4).
At the end of each session, clean the device with a soft dry
cloth or tissue, making sure to remove all of the ultrasound gel.
After cleaning, verify the product is intact. Discontinue
use with any sign of damage or loss of cover integrity.
Once clean and dry, store the device in its carry case
(g. 5) and locate it with its accessories in a safe cool an
dry place, away from direct sunlight. Follow temperature
and humidity guidelines as specied in this Instructions for Use.
Disinfection
The device is intended for personal use. If transferred between
patients, it must be disinfected. In any case of need for disinfection,
follow the instructions below:
1.
Before cleaning or disinfection, ensure that it is unplugged
from the power supply.
2.
Use a soft cloth, mildly moistured with soap
or detergent solution, to clean the device
until it is visually clean.
3.
Use a soft cloth, mildly moistured with
water to remove soap or detergent
solution residuals.
4.
Use a soft, dry cloth to dry the device.
5.
Use a soft cloth, moistened with 70%
medical grade alcohol, to disinfect the
device for at least 1 minute.
6.
Use a soft, dry cloth to dry the device.
7.
Verify the device is intact. Discontinue use
with any sign of damage or loss of cover
integrity.
8.
Once clean and dry, store the device
with its accessories in a safe cool
and dry place, away from direct sunlight.

18
Troubleshooting
Problem: The device failed to detect the Fetal Heart Rate,
or measured rate is inconsistent.
Details: The device failed to detect the Fetal Heart Rate at
all recommended locations.
Solution:
1. Measurement is fully eective when:
a. There is always a thin and even layer of gel
between HeraBEATTM and your skin. Note that the
gel might dry after several minutes. Add gel as
required to maintain a thin, moist layer.
b. Apply a small amount of pressure on HeraBEATTM
against your skin. Verify full contact during the
entire measurement.
c. Hold HeraBEATTM steady while it is searching and
measuring.
d. Carefully follow the step-by-step guidance as the
App suggests.
2. Excessive or prolonged fetal, maternal or device
movements can degrade the ability to locate and
measure the Fetal Heart Rate (FHR). If you’re
experiencing frequent fetal movements during the
measurement, stop the measurement and try again
once fetal movements have stopped.
3.
Detection of Fetal Heart Rate in early weeks of
pregnancy can be challenging in some cases. The size of
the fetus’ heart can be very small and it might be missed.
4. It is recommended to perform measurements at
least 1 hour after major meals as these may cause
abdominal noises that may interrupt the measurement.
5. Consult with your physician.
Problem:
Device is ashing a white light 3 times and turns OFF.
Details: Device may require charging
Solution:
1. Charge the device.
2. If error persists, contact service.
(support@hera-med.com)
Problem: App crashes.
Details: A general error may have occurred.
Solution:
1. Close and restart the app.
2. If error persists, restart the smartphone.
3. If error persists, contact service.
(support@hera-med.com)
Problem: Charge light is ashing both orange and green lights.
Details: An error with the charge circuit has occurred.
This may be due to a depleted battery.
Solution:
1. Contact service. (support@hera-med.com)
Problem: The device does not turn ON or
the device’s battery is low.
Details:
The ‘low battery’ message is displayed.
Solution:
1.
Stop the current test using END button
.
2. Charge the device.
Note: If the battery does not charge or does
not hold a charge, please contact Service.
(support@hera-med.com)
19
Smartphone General Security and Privacy
The product software installed and the information stored
on the smartphone should be protected from unauthorized
access and from external threats.
We recommend the following security measures:
The physical protection of the device:
Do not leave the device and your smartphone unattended;
Do not allow others to use your devices.
Use only ocial Operating System versions. Do not jailbreak
or root smartphones;
Avoid installing untrusted apps on the smartphone;
Download apps only from trusted sources such as the
Google Play store.
Minimize installation of unnecessary apps;
Install latest software versions and apply security patches
on the smartphone;
Beware of wireless connections: avoid connection to
untrusted services, and disable auto-connect to Wi-Fi
signals;
Avoid making your smartphone avaible in networking
(Bluetooth or Wi-Fi);
Hide the network SSID (network name);
Avoid use of untrusted information services, web services
and web apps (do not follow links sent in suspicious emails
or text messages);
Access controls: use of screen lock and secured unlock
option is recommended (numeric PIN code, password or
nger print);
Security software including malware protection is
recommended;
Use ocial ;antitheft services;
Backup smartphone data.
Log o u t of any w e b s i t e that y o u co n d u c t n a n c i a l transac tions
on.
Special care should be taken when using smar tphones in public
places and other unprotected areas. Protection should be in
place to avoid the unauthorized access to or disclosure of the
information stored and processed by the smartphone, e.g.
using cryptographic techniques and enforcing use of secret
authentication information.
The smartphone should also be physically protected against
theft especially when left, for example, in cars and other
forms of transportation, hotel rooms, conference centers
and meeting places. Devices carrying important, sensitive or
personal health information should not be left unattended
and, where possible, should be physically locked away, or
special locks should be used to secure the devices. Use of
a home Wi-Fi system with secured access (via Wi-Fi WPA2
standard) is strongly recommended.
The practical implementation of technical security elements
varies by module of smartphone and version of operating
system and security software used, and may employ several
technologies, including virus scanning software, authentication
technologies, etc.
The information recorded by the app is stored on your
smartphone. If someone gains access to your smartphone,
they can be exposed to this information.
Sharing the information via the app, is at your own risk and
discretion.

20
Auto Acquisition stop 5 minutes of successful measurement
Recommended Ultrasound Water soluble hypoallergenic
Transmission Gel ultrasonic gel
Power consumption < 2 W
Rechargeable Li-ion Battery
Nominal Capacity 3.7VDC, 1250 mAh
Continuous Work Time 4 hours (with a new battery)
Power Input 5 V DC, >0.3A
Charge Time 4 hours
Ultrasound
Nominal Frequency 2 MHz ±10%
Ultrasonic Output Power (P) 70 mW
Peak acoustic pressure
(pr, at Z MI) 0.022 MPa
Ultrasonic Output Intensity
(Isat ) <20 mW/cm2
Mechanical Index (MI) 0.022
Thermal index (TIS; TIB) 0.26; 0.7
Measurement Mode Continuous Wave Ultrasound Doppler
Eective Radiating Area
of Transducer 4.9±0.5 cm2
HeraBEAT™ Specication
Safety
Complies with IEC/EN 60601-1, 60601-1-2,
60601-1-11, 60601-2-37
Classication
Anti-electric Shock Type Internally powered equipment
Anti-electric Shock Degree Type BF equipment
Degree of Protection against
Harmful Ingress of Water IP22
Protection against falling drops
of water when unit is tilted 15o
Physical Characteristics
Device size 88 x 37 mm, 3.5 x 1.5 inch
(Diameter × Height, ±2mm)
Device weight Approximately 130g (.29 lb.)
Operating Environment
Temperature From 5°C to 40°C (41°F up to 104°F)
Humidity From 5% up to 90% RH
(non-condensing)
Storage/Transport Environment
Temperature
From -20°C to +60°C (-4°F up to 140°F)
Humidity From 5% up to 95% (non-condensing)
Light intensity No direct sun light
FHR Performance
Pregnancy Week 12 to 42
FHR Measuring Range;
Accuracy; Resolution 50 to 240 BPM; ±2BPM; 1 BPM
Other manuals for HeraBEAT
1
Table of contents