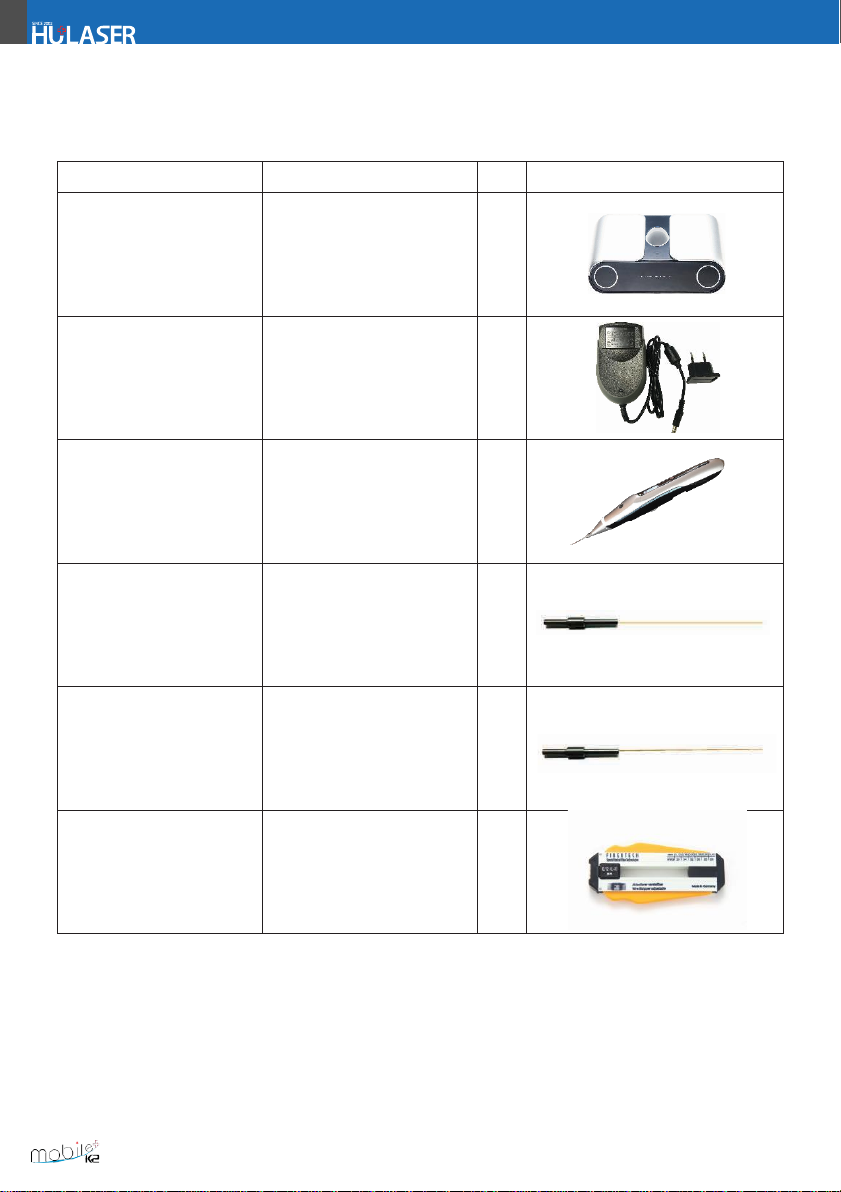
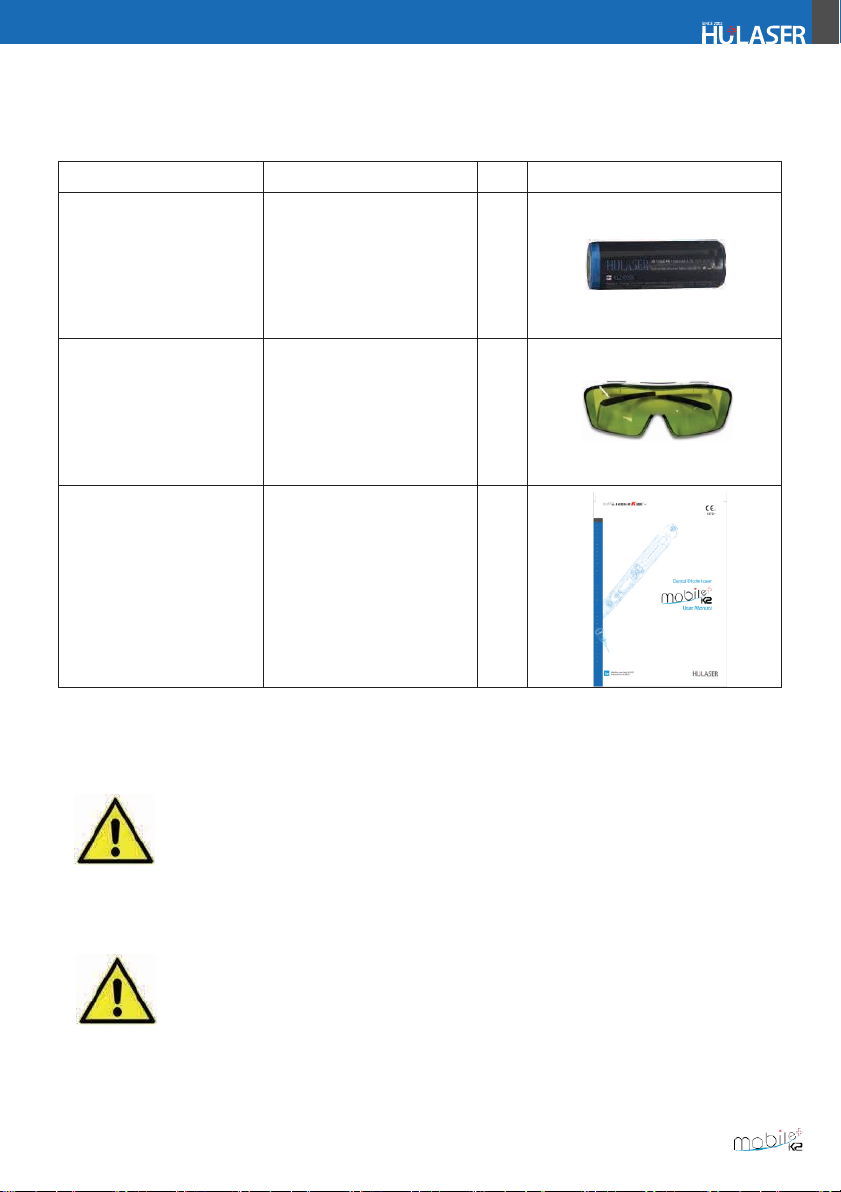
1.
SAFETY INFORMATION
The purpose of the safety information is to ensure the safety of users and prevent property
damage. Please read this document carefully for proper use.
1-1. Safety information
Danger
DANGER indicates a hazardous situation which, if not avoided,
could result in serious injuries.
●
Pointing the beam directly to the eyes may cause damage to the eye. Do not
use it close to the eyes of people orpets.
●
Do not use the device in potentially explosive environments and flammable apparatuses
or oxidizing gases such as nitrous oxide(N
2
O) and oxygen(O
2
). When using flammable solutions
or adhesives for cleaning and disinfecting, be aware of the ignition of gases.
Warning
WARNING indicates a hazardous situation which, if not avoided,
could result in moderate or minor injuries.
●
When the device is not working properly, stop using thedevice.
●
Thefibertip is supplied in non-sterileandmust be sterilizedbefore use.Follow thesterilizing
instruction according to the chapter3.
Caution
CAUTION indicates a hazardous situation which, if not avoided,
could result in moderate or minor injuries.
●
Doctor, assistant and all others inside the operatory must wear the laser protective eyewear for the
laser wavelength of 980nm. The patient completely covers the eyes with a cloth. If not, wear goggles
and close your eyes. The laser protective eyewear needs to be inspected periodi- cally for your safety.
●
Do not point the laser beam to metallic or reflective objects like mirrors as the laser may reflect and
cause a hazardous situation.
●
Do not look directly into the beam, laser aperture or at specular reflections.
Never direct or point the beam at anyone’s eyes.
This must be followed even when wearing safety goggles.
Likewise, do not look directly into the laser or point it at others' eyes while wearing safety goggles.
4