International Biomedical AirBorne NxtGen Transport Incubator User manual


P
h
F
a
E-
M
We
M
a
In
t
8
2
A
u
U
S
A
u
E
m
P
r
2
5
T
h
h
one: (5
1
a
x: (512)
M
ail: sa
l
e
bsite:
h
a
iling ad
d
t
ernation
2
06 Cros
s
u
stin, TX
S
A
u
thorize
d
m
ergo E
u
r
insesse
g
5
14 AP
h
e Hague
,
S
1
2) 873-0
0
873-909
0
l
es@int-
b
h
ttp://ww
w
d
ress:
al Biom
e
s
Park D
r.
78754
d
represe
n
u
rope
g
racht 20
,
The Ne
t
S
erv
i
0
33
0
b
io.com
w
.int-bio
.
e
dica
l
r.
n
tative i
n
t
herland
s
i
ce
M
.
com
n
Europe
s
M
anu
a
for Reg
u
al
u
latory A
f
f
fairs:

This page intentionally left blank.

TABLE OF CONTENTS
Part No. 715-0132, Rev. C - 1 -
1. GENERAL INFORMATION ....................................................................................................... 15
1.1. Introduction ................................................................................................................... 15
1.2. Intended Use ................................................................................................................. 15
1.3. Classification ................................................................................................................ 15
1.4. Safety Summary ............................................................................................................ 15
1.5. Safety Notice ................................................................................................................. 16
1.6. Important Safety Considerations ................................................................................. 16
1.7. Symbols ......................................................................................................................... 28
2. INITIAL SETUP ......................................................................................................................... 30
2.1. Unpacking Instructions ................................................................................................ 30
2.2. Mounting Provisions .................................................................................................... 31
3. SYSTEM OVERVIEW ............................................................................................................... 32
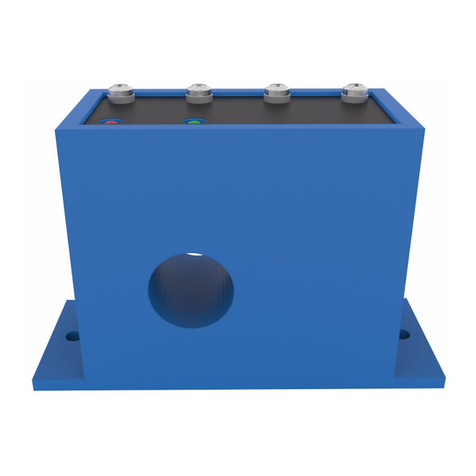
3.1. Physical Description ..................................................................................................... 32
3.1.1. Transport Incubator ........................................................................................... 32
3.1.2. Rear Electronics Panel ...................................................................................... 33
3.1.3. Patient Connector Panel (Located on left or right end panel) ........................ 34
3.2. System Power ............................................................................................................... 34
3.2.1. AC Power ............................................................................................................ 34
3.2.2. DC Power ............................................................................................................ 34
3.2.3. Battery Power ..................................................................................................... 35
3.3. External Power Connections ....................................................................................... 35
3.3.1. External AC Power ............................................................................................. 35
3.3.2. External DC Power ............................................................................................. 36
3.3.3. Multiple Socket Outlets ..................................................................................... 36
3.3.4. DC Power Outlet ................................................................................................. 37
4. PRE-USE CHECKOUT ............................................................................................................. 37
4.1. Pre-Use Checkout ......................................................................................................... 37
4.1.1. Operational Check ............................................................................................. 37
4.1.2. Infant Chamber Check ....................................................................................... 38
4.1.3. Accessories ........................................................................................................ 38
4.1.4. Sensors and Cables ........................................................................................... 38
5. OPERATING INSTRUCTIONS ................................................................................................. 38
5.1. Power On ....................................................................................................................... 38
5.2. Power On Self-Test ....................................................................................................... 38
5.3. Pre-Heat Mode ............................................................................................................... 39
5.4. Administrator Menu ...................................................................................................... 39
5.5. Service Menu ................................................................................................................. 44
6. NORMAL OPERATION............................................................................................................. 49
6.1. Main Screen Navigation ............................................................................................... 50
6.2. Thermal Operation ........................................................................................................ 52
6.3. Thermal Control System Description .......................................................................... 53

TABLE OF CONTENTS
Part No. 715-0132, Rev. C - 2 -
6.4. Setup .............................................................................................................................. 53
6.4.1. Skin Temperature Probes .................................................................................. 53
6.5. Admitting a Patient ....................................................................................................... 60
6.5.1. Patient Positioning Straps Installation and Application ................................. 61
7. MESSAGE CENTER ................................................................................................................. 62
7.1. Indicators ....................................................................................................................... 62
7.2. Power off the Device ..................................................................................................... 64
8. GENERAL ALARM INFORMATION ......................................................................................... 65
8.1. Alarm Types .................................................................................................................. 65
8.2. Alarm Dropdown ........................................................................................................... 67
8.3. Alarm Audio Pause ....................................................................................................... 68
8.4. Technical Error ............................................................................................................. 68
8.5. Alarm Verification ......................................................................................................... 69
8.5.1. Test of Temperature Alarms .............................................................................. 69
8.5.2. Test of SpO2 Alarms........................................................................................... 69
8.5.3. Test of O2 Alarms ............................................................................................... 70
8.5.4. Test of Heated Mattress Alarms ........................................................................ 70
9. DATA STORAGE ...................................................................................................................... 70
9.1. Introduction ................................................................................................................... 70
9.2. Setup .............................................................................................................................. 71
9.3. General Operation ......................................................................................................... 71
10. PULSEOX (OPTIONAL)............................................................................................................ 71
10.1. Introduction ................................................................................................................... 71
10.2. Pulse Oximetry Theory of Operation ........................................................................... 72
10.3. Setup .............................................................................................................................. 73
10.4. General Operation ......................................................................................................... 73
10.5. Setting Alarm Limits - %SpO2 ...................................................................................... 75
10.6. Setting Alarm Limits - Pulse Rate ................................................................................ 76
10.7. Signal Strength ............................................................................................................. 77
10.8. Perfusion Index (Masimo Only) ................................................................................... 77
10.9. Pulse Rate (BPM) .......................................................................................................... 77
10.10. Sensors .......................................................................................................................... 78
10.11. Pulse Oximetry Testers / Simulators ........................................................................... 78
11. PHOTOTHERAPY ..................................................................................................................... 78
11.1. Introduction ................................................................................................................... 78
11.2. Setup .............................................................................................................................. 79
11.2.1. Check Intensity ................................................................................................ 79
11.2.2. Prepare the Infant ............................................................................................ 79
11.3. General Operation ......................................................................................................... 79
11.3.1. Effective Surface Area .................................................................................... 81
11.3.2. Phototherapy Light Lifetime Indicator ........................................................... 82

TABLE OF CONTENTS
Part No. 715-0132, Rev. C - 3 -
12. OBSERVATION LIGHT............................................................................................................. 82
12.1. Introduction ................................................................................................................... 82
12.2. Setup .............................................................................................................................. 82
12.3. General Operation ......................................................................................................... 83
12.3.1. Turning the Observation Light ON ................................................................. 84
12.3.2. Turning the Observation Light OFF ............................................................... 85
12.3.3. Adjusting the Observation Light Intensity .................................................... 85
12.3.4. Observation Light System Interactions with the Optional
Phototherapy System ...................................................................................... 85
13. AMBIENT OXYGEN MONITOR ................................................................................................ 85
13.1. Introduction ................................................................................................................... 85
13.1.1. Temperature ..................................................................................................... 85
13.1.2. Pressure ........................................................................................................... 86
13.1.3. Humidity ........................................................................................................... 86
13.1.4. Oxygen Sensors .............................................................................................. 86
13.2. Setup .............................................................................................................................. 86
13.2.1. Calibration ........................................................................................................ 87
13.3. General Operation ......................................................................................................... 88
14. HEATED MATTRESS ............................................................................................................... 89
14.1. Introduction ................................................................................................................... 89
14.2. Setup .............................................................................................................................. 89
14.3. General Operation ......................................................................................................... 90
15. SUCTION .................................................................................................................................. 91
15.1. Introduction ................................................................................................................... 91
15.2. Setup .............................................................................................................................. 91
15.3. General Operation ......................................................................................................... 92
16. TIMERS ..................................................................................................................................... 94
16.1. Introduction ................................................................................................................... 94
16.2. General Operation ......................................................................................................... 94
17. DEVICE SETTINGS .................................................................................................................. 97
17.1. Introduction ................................................................................................................... 97
17.2. General Operation ......................................................................................................... 97
18. CLEANING .............................................................................................................................. 100
18.1. Introduction ................................................................................................................. 100
18.2. Inspection .................................................................................................................... 100
18.3. Cleaning of Oxygen Sensor (Optional Equipment) .................................................. 102
18.4. Cleaning of Cables (Optional Equipment) ................................................................ 102
18.5. Cleaning of Patient Temperature Probes .................................................................. 102
18.6. Cleaning of Pulse Ox Sensor (Optional Equipment) ................................................ 102
18.7. Cleaning of Mattress/Heated Mattress ...................................................................... 103
18.8. Cleaning of Suction .................................................................................................... 103
19. PREVENTATIVE MAINTENANCE .......................................................................................... 103

TABLE OF CONTENTS
Part No. 715-0132, Rev. C - 4 -
19.1. Introduction ................................................................................................................. 103
19.2. Pre-Use Checkout ....................................................................................................... 103
19.2.1. Operational Check ......................................................................................... 103
19.2.2. Infant Chamber Check .................................................................................. 104
19.2.3. Mattress Tray Check ..................................................................................... 104
19.2.4. Light Bar ......................................................................................................... 104
19.2.5. Accessories ................................................................................................... 104
19.2.6. Sensors and Cables ...................................................................................... 105
19.3. Monthly Maintenance ................................................................................................. 105
19.3.1. Infant Chamber Inspection and Cleaning .................................................... 105
19.3.2. Air Flow System Inspection and Cleaning .................................................. 105
19.3.3. Phototherapy ................................................................................................. 105
19.4. Annual Maintenance ................................................................................................... 105
19.4.1. Alarm Verification .......................................................................................... 105
19.4.2. Airflow Tray Filter Replacement ................................................................... 106
19.4.3. Suction Module Functional Check ............................................................... 106
19.5. Battery Maintenance ................................................................................................... 107
19.5.1. Battery Removal ............................................................................................ 107
19.6. Calibration Schedule .................................................................................................. 107
19.7. Phototherapy Light Lifetime Indicator ...................................................................... 108
20. TROUBLESHOOTING ............................................................................................................ 108
20.1. General ........................................................................................................................ 108
20.2. Power On Self-Test ..................................................................................................... 109
21. ACCESSORIES ...................................................................................................................... 109
22. INTERNAL COMPONENT ACCESS ...................................................................................... 114
22.1. Electronics Location and Description ....................................................................... 115
22.2. Electronics Compartment Access ............................................................................. 115
22.3. Power Module Removal .............................................................................................. 117
22.4. Touch Screen Assembly Removal ............................................................................ 118
22.5. Knob Assembly Removal ........................................................................................... 119
22.6. Suction Removal ......................................................................................................... 120
22.7. Connector Bay Removal ............................................................................................ 120
22.8. PulseOx Sensor Board Removal (Optional Equipment) .......................................... 120
22.9. Auxiliary Medical Devices .......................................................................................... 121
23. REPAIR POLICY ..................................................................................................................... 121
24. WARRANTY ............................................................................................................................ 122
25. SCHEMATICS ......................................................................................................................... 123
Appendix A Specifications .................................................................................................... 125
Operating, Storage, and Transport Environment ............................................................... 125
General Mechanical Specifications ...................................................................................... 125
Electrical Specifications ....................................................................................................... 126

TABLE OF CONTENTS
Part No. 715-0132, Rev. C - 5 -
Operational Specifications ................................................................................................... 126
Pulse Oximeter Specifications (Optional Feature) ............................................................. 126
Range ............................................................................................................................ 126
Resolution ..................................................................................................................... 127
Sensor Peak Wavelengths ........................................................................................... 127
Sensor Maximum Power Output ................................................................................. 127
Masimo Sensor Accuracy ............................................................................................ 128
Nellcor Sensor Accuracy ............................................................................................. 131
Interfering Substances ................................................................................................ 133
Ambient Oxygen Monitor Specifications (Optional Feature) ............................................. 133
Interfering Substances ................................................................................................ 133
Phototherapy Specifications ................................................................................................ 134
Observation Light Specifications ......................................................................................... 134
Suction Specifications .......................................................................................................... 134
Appendix B EMC Specifications ........................................................................................... 135
Appendix C Essential Performance ...................................................................................... 139
Appendix D Alarms and Alerts .............................................................................................. 140
Appendix E Product Disposal/Recycle ................................................................................ 145
Environmental Requirements ............................................................................................... 145
Appendix F Error Codes ........................................................................................................ 146

TABLE OF CONTENTS
Part No. 715-0132, Rev. C - 6 -
This page intentionally left blank.

Pa
r
r
t No. 715-01
3
3
2, Rev. C
L
A
A
NGUA
G
G
E DI
S
- 7 -
S
CLAI
M
M
ER

Pa
r
r
t No. 715-01
3
3
2, Rev. C
L
A
A
NGUA
G
G
E DI
S
- 8 -
S
CLAI
M
M
ER

Pa
r
r
t No. 715-01
3
3
2, Rev. C
L
A
A
NGUA
G
G
E DI
S
- 9 -
S
CLAI
M
M
ER

Pa
r
r
t No. 715-01
3
3
2, Rev. C
L
A
A
NGUA
G
G
E DI
S
- 10 -
S
CLAI
M
M
ER

Pa
r
r
t No. 715-01
3
3
2, Rev. C
L
A
A
NGUA
G
G
E DI
S
- 11 -
S
CLAI
M
M
ER

Pa
r
r
t No. 715-01
3
3
2, Rev. C
L
A
A
NGUA
G
G
E DI
S
- 12 -
S
CLAI
M
M
ER

Pa
r
r
t No. 715-01
3
3
2, Rev. C
L
A
A
NGUA
G
G
E DI
S
- 13 -
S
CLAI
M
M
ER

LANGUAGE DISCLAIMER
Part No. 715-0132, Rev. C - 14 -
This page intentionally left blank.

Part No. 715-0132, Rev. C - 15 -
1. GENERAL INFORMATION
1.1. Introduction
This service manual describes the system, setup, operation, cleaning, maintenance,
troubleshooting, and technical specifications for the NxtGen Transport Incubator. Read
the NxtGen manual thoroughly to understand all instructions, warnings, cautions, and
notes before operating the device. International Biomedical is not responsible for any
malfunction due to improper use or service by unauthorized International Biomedical
personnel. For any technical problem, contact your International Biomedical
representative. There are no known contraindications associated with the NxtGen
Transport Incubator.
1.2. Intended Use
The NxtGen Transport Incubator is intended for use by personnel trained in neonatal
care to facilitate the movements of neonates by air or ambulance. The Transport
Incubator provides heat in a controlled manner to neonates through an enclosed
temperature controlled environment. The Transport Incubator is also intended to carry
equipment designed for airway management and monitoring of the neonatal infants
status. The device provides two modes of heat: Manual (Operator) Controlled or Skin
(Servo) Controlled. All Transport Incubators may be optionally configured with pulse
oximetry, a suction device, and an integrated heated mattress. In addition, the NxtGen
Transport Incubator may be configured with optional blue LED phototherapy to treat
indirect hyperbilirubinemia.
1.3. Classification
According to the standard IEC 60601-1 of the International Electrotechnical Commission,
Medical electrical equipment, Part 1: General requirements for safety, the infant
Transport Incubator is classified as follows:
Class II / Internally Powered, according to the type of protection against electric
shock
The NxtGen Transport Incubator and its applied parts are Type BF equipment. T1
and T2 patient probes, Pulse oximeter probe, ambient oxygen sensor, and the
mattresses are applied parts. Care must be taken that additional equipment
connected to the baby is electrically safe. To ensure patient electrical isolation,
connect only to other equipment with electronically isolated circuits.
IP33, according to the degree of protection against harmful ingress of water
Equipment is not suitable for use in the presence of a flammable anesthetic
mixture with air or with oxygen or nitrous oxide.
Continuous operation for the mode of operation
1.4. Safety Summary
The NxtGen Transport Incubator is intended to be used by trained clinicians and
operated in a manner consistent with the instructions contained in this manual. Refer to
any additional training, procedures, requirements, or documentation beyond those
identified here for operation and policies required within the institution. All personnel
operating the Transport Incubator must be familiar with the warnings and operating
procedures contained in this manual. International Biomedical is not to be held
responsible if the Transport Incubator is used in a manner inconsistent with the
instructions herein.

Pa
r
T
h
to
g
G
e
O
B
m
e
r
t No. 715-01
3
1.5.
1.6.
NOTE
S
h
e principal
g
ether her
e
e
neral
B
SERVE B
e
dical proc
e
3
2, Rev. C
A
ny serio
u
Internatio
n
Safety No
t
The Tran
s
electroma
g
this equip
m
interferen
c
interferen
c
Transport
Important
Within cer
t
be labele
d
equipmen
t
A
ssembly
service lif
e
additional
having th
e
S
:
WARNIN
G
e
for emph
a
EST PRA
C
e
dures or
s
u
s incident
t
n
al Biomed
i
t
ice
port Incub
a
g
netic inte
r
f
m
ent can r
a
c
e to other
d
c
e from oth
e
Incubator t
Safety Co
n
t
ain gover
n
d
by an app
t
, risk (leak
a
of a Medic
a
e
require e
v
pertinent i
n
e
following
s
G
and CAU
a
sis.
C
TICE: Th
e
s
taff prefer
e
t
hat occur
s
i
cal and th
e
a
tor has be
e
f
erence an
d
a
diate radi
o
d
evices. T
e
r devices.
o reduce o
n
sideration
s
n
mental juri
s
roved testi
n
a
ge) curre
n
a
l Electric
a
v
aluation to
n
formation
w
s
ignificanc
e
TION notic
e
instructio
n
e
nce conce
- 16 -
s
in relation
e
compete
n
e
n tested
a
d
suscepti
b
o
frequenc
y
he Transp
o
If RF inte
r
r eliminate
s
s
dictions,
a
n
g laborat
o
n
t and grou
a
l System a
the requir
e
w
ill be disp
e
:
t
t
es to be o
b
n
s in this
m
rning patie
n
to this dev
n
t authority
a
nd found t
o
b
ility as def
y
(RF) ener
g
o
rt Incubat
o
r
ference is
the effects
a
ll intercon
n
o
ry. After i
n
nding requ
nd modific
a
e
ments of
6
layed usin
g
Maintena
n
technique
personal i
carefully f
o
Maintenan
c
technique,
patient har
not careful
l
Maintenan
c
technique,
essential t
o
b
served in
u
m
anual in n
o
nt care.
v
ice should
of the app
r
o
comply
w
f
ined by IE
C
g
y and ma
y
o
r may als
o
suspected
,
.
n
ected acc
e
n
terconnec
t
irements
m
a
tions to th
i
6
0601-1.
S
g
warnings
,
n
ce or ope
r
, etc., whi
c
njury or l
o
ollowed.
c
e or oper
a
etc., whic
h
m or dama
l
y followed
.
c
e or oper
a
etc., whic
h
o
emphasi
z
u
se of this
d
o
way supe
be reporte
d
r
opriate m
e
w
ith limits f
o
C
60601-1-
2
y
cause ha
r
o
be affect
e
,
relocate
o
e
ssory equ
t
ion with a
c
m
ust be mai
i
s device d
u
S
afety conc
e
,
cautions,
a
r
ating pro
c
c
h may re
s
o
ss of life i
f
a
ting proce
d
h
may resul
t
ge to equi
p
.
a
ting proce
d
h
is consid
e
z
e.
d
evice are
rsede esta
d
to
e
mber state
o
r
2
. Howev
e
r
mful
e
d by
o
r shield th
e
ipment mu
s
c
cessory
i
ntained.
u
ring the
e
rns or
a
nd notes,
c
edure,
s
ult in
f
not
d
ure,
t
in
p
ment if
d
ure,
e
red
brought
blished
.
e
r,
e
s
t

Part No. 715-0132, Rev. C - 17 -
As with all medical equipment, carefully route patient cabling to reduce the possibility of patient
entanglement or strangulation.
Do not modify this equipment without proper authorization from International Biomedical.
The NxtGen Transport Incubator should be used by appropriately trained personnel and under the
direction of qualified medical staff familiar with currently known risks and benefits of the NxtGen
Transport Incubator use.
Skin temperature probe is not a rectal probe. The skin temperature sensor is not to be used as a
rectal probe.
Warming transdermal medications can increase drug delivery and may result in patient danger.
Do not use the observation light, the pulse oximeter, heated mattress, or ambient oxygen monitor in
the presence of flammable anesthetics or other flammable substance in combination with air, oxygen-
enriched environments, or nitrous oxide.
USE OF OXYGEN INCREASES FIRE DANGER: Spark-producing auxiliary equipment should not be
placed in or near the Transport Incubator.
USE OF OXYGEN INCREASES FIRE DANGER: Small amounts of flammable agent left in the
incubator can cause fire.
Avoid direct sunlight or radiant heat, which can cause a dangerous increase in chambers air
temperature and affect the amount of irradiation being provided to the patient.
The use of oxygen may increase the noise level within the infant chamber.
Use of this equipment adjacent to or stacked with other equipment should be avoided because it could
result in improper operation. If such use is necessary, this equipment and the other equipment should
be observed to verify that they are operating normally.
Use of accessories, transducers, and cables other than those specified or provided by the
manufacturer of this equipment could result in increased electromagnetic emissions or decreased
electromagnetic immunity of this equipment and result in improper operation.
Varying ambient conditions, such as the ambient temperature and/or different radiation sources, may
adversely affect the patient. Please refer to your institutions policy and procedure regarding
appropriate ambient conditions.
Portable RF communications equipment (including peripherals such as antenna cables and external
antennas) should be used no closer than 30 cm (12 inches) to any part of the NxtGen system,
including cables specified by the manufacturer. Otherwise, degradation of the performance of this
equipment could result.
Do not use the NxtGen system during magnetic resonance imaging (MRI) scanning. The NxtGen
system may affect the MRI image and the MRI unit may affect the NxtGen systems operation.
Do not use liquids in or around the Transport Incubator.
Table of contents
Other International Biomedical Accessories manuals