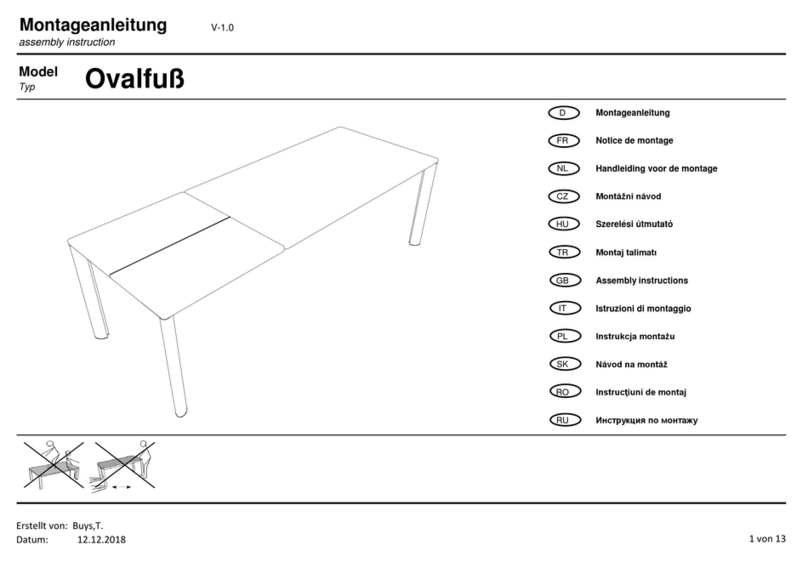
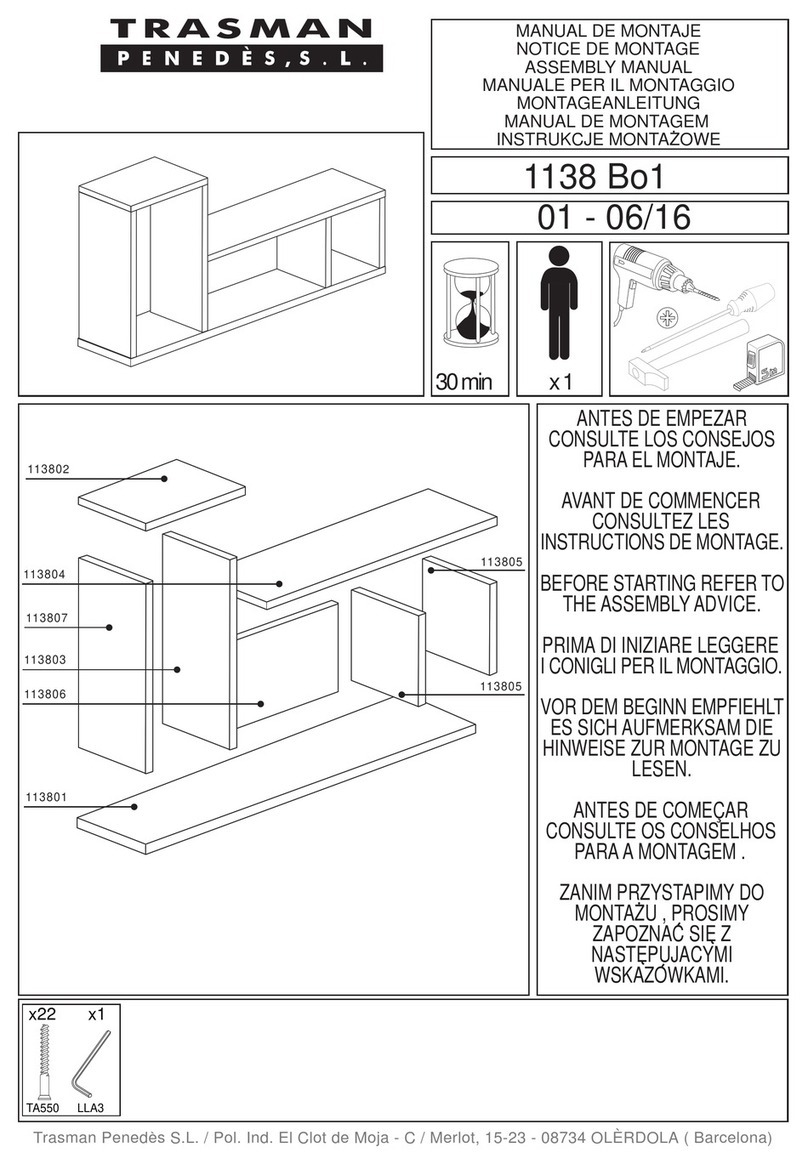
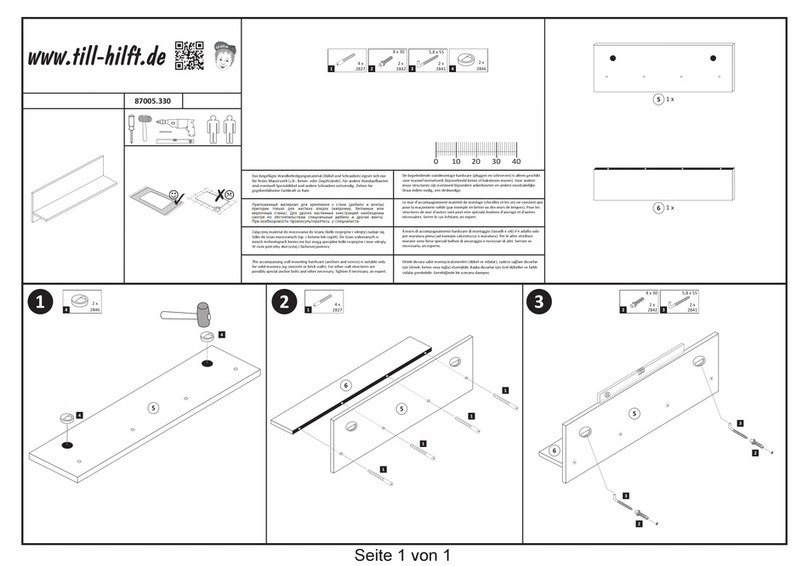
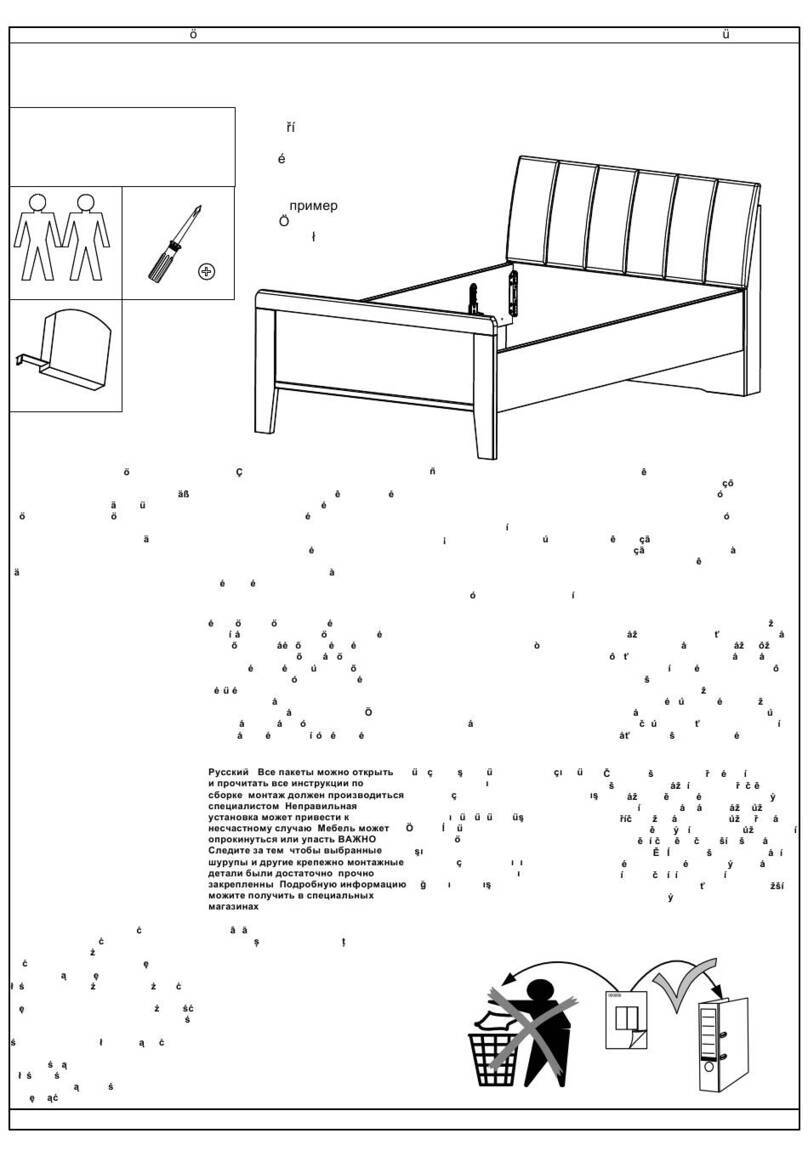
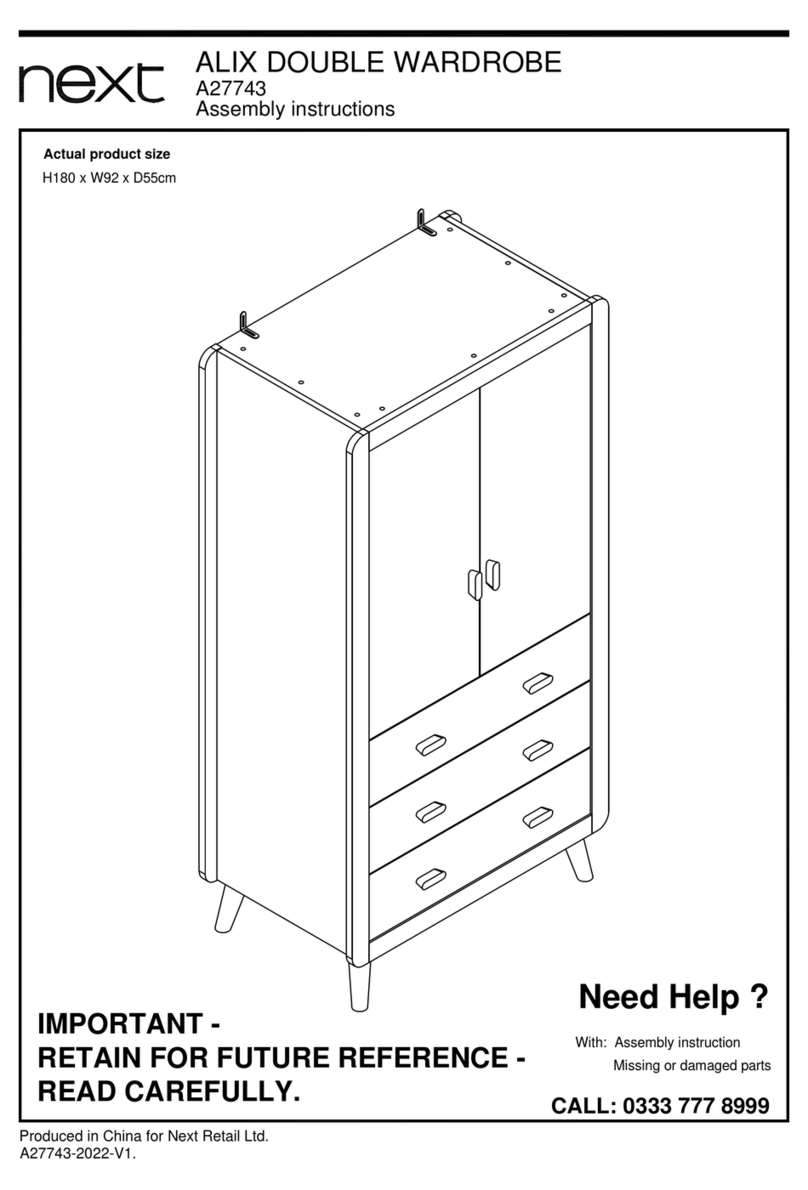
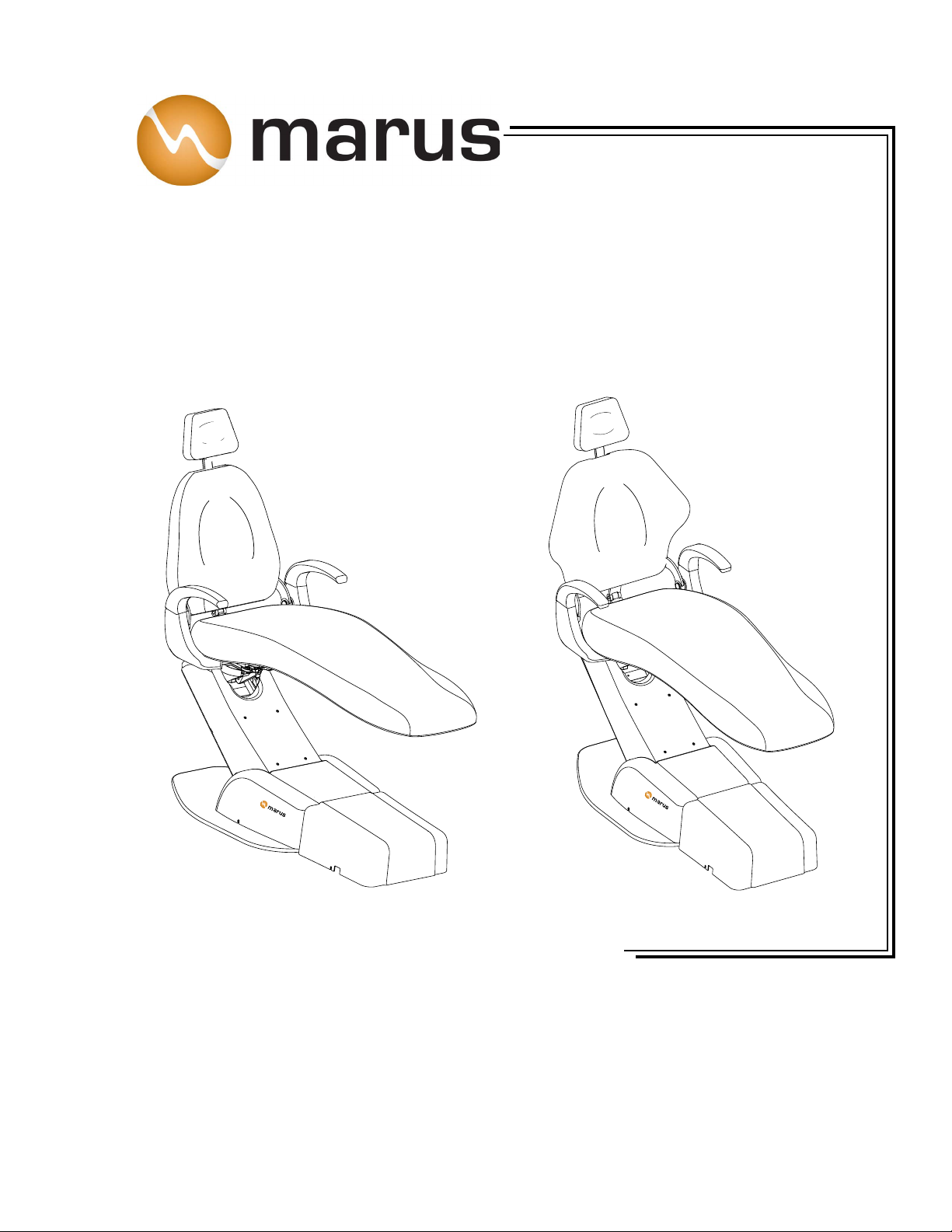
5
31064 R00
WARNING: *The Manufacturer makes no
representation as to the disinfectant efcacy
of these products. We make no warranty
expressed or implied that these disinfectants
will not damage the surface nishes. Damage
and discoloration of the surface nishes are
not covered under the warranty.
Strong Phenols/Phenol Alcohol combinations
Sodium Hypochlorite/Household Bleach
Sodium Bromide
Strong Alcohol
Household Cleaners (Dental Equipment Only)
Citric Acids
Iodophors**
Ammonium Chloride
Accelerated Hydrogen Peroxide (0.5%)
CLEANING, DISINFECTING & STERILIZATION
IMPORTANT: Do not use powdered cleansers, scouring pads or abrasive scrubbers on any of
the painted, plastic or metal surfaces of this dental unit. To remove dried-on material, use a soft-
bristled brush and a solution of mild detergent.
Equipment can be cleaned with a solution of mild detergent and warm water. A variety of surface disinfectants are
available for use in dental treatment rooms. Some of these can cause discoloration of painted, plated or anodized
surfaces with repeated use. This can be minimized by careful adherence to the disinfectant manufacturer’s
instructions and by frequent washing with soap and water.
The Manufacturer strongly advocates the barrier
technique be used whenever possible to preserve
the nish and appearance of the equipment. Wher-
ever possible disposable barriers should be used
and changed between patients. The barrier tech-
nique will ensure maximum long term durability of
the surfaces and nishes of the equipment.
Unacceptable Disinfectants Conditionally Acceptable Disinfectants*
These disinfectants will harm the surface nishes of
dental equipment and are not recommended. Use of
these products will void your warranty.
These disinfectants have been found to be the
least harmful to the equipment surfaces by our test
methods.
Disinfection & Sterilization
Infection Control in the dental ofce continues to
be a high priority for our customers and end users.
OSHA, the ADA and the CDC are also involved in this
complex issue. The Manufacturer will not attempt to
specify the required intervals for disinfection nor can
it recommend the overall best surface disinfectant.
Please refer to the Infection Control Recommendations
published by the American Dental Association for further
information. The question is often asked, “What should
I use to disinfect my dental unit, chair and light?” This
question is more complex than it seems because of
the wide variety of products on the market as well as
formulations of the products changing to meet the
needs of increased asepsis.
Barrier Technique
Chemical Disinfection
Regardless of the chemical disinfectant used, it
is imperative that the equipment be thoroughly
washed with mild soap and warm water at least
once per day. This wash-down will minimize the
harmful effects of chemical disinfectant residues
being allowed to accumulate on the equipment.
When using chemical disinfectants, always pay
strict attention to the disinfectant manufacturer’s
directions. When using concentrated disinfectants,
measure the concentrate carefully and mix
according to package directions. Disinfectant
solutions that are relatively harmless to surfaces at
their recommended strengths can be corrosive at
higher than recommended dilution ratios.
WARNING: Disinfect only by wiping,
no spray disinfection. Please be
aware that the manufacturer expressly
rejects any claims for warranty or
damages when using other cleaning
and disinfection solutions.
**Iodophor-based disinfectants will cause yellow staining on many surfaces.
Quaternary Ammonium
Chemical Composition
Chemical Composition