Merit Medical MetaSTAR 3195 User manual

403321001_001 2016-08-31
OPERATOR’S MANUAL
MetaSTAR™ RF Generator
Catalog No. 3195
Manufactured By:
Merit Medical Systems, Inc.
1600 West Merit Parkway
South Jordan, Utah 84095 U.S.A.
1-801-253-1600
U.S.A. Customer Service 1-800-356-3748
Authorized Representative:
Merit Medical Ireland Ltd.
Parkmore Business Park West
Galway, Ireland
+31 43 358 82 22
P a t e n t s p e n d i n g
CAUTION: Federal law (USA) restrictsthis deviceto sale by or onthe order of a physician.

403321001_001 2016-08-31
Disclaimer Merit Medical Systems, Inc. reserves the right to change its products at any time in order to incorporate
the most recent technological developments. This guide is subject to change without notice.
Although this guide has been prepared with every regard to insure the accuracy of its contents, Merit
Medical Systems, Inc. assumes no liability for any damages incurred from the application of this
information. The recommendations are designed to serve as a general guideline. They are not intended
to supersede institutional protocols or professional clinical judgment concerning patient care.
Table of Contents
Section Page
1. BRIEF DEVICE DESCRIPTION:................................................................................................. 3
2. INDICATIONS FOR USE:........................................................................................................... 3
3. CONTRAINDICATIONS:............................................................................................................. 3
4. WARNINGS:............................................................................................................................... 3
5. PRECAUTIONS:......................................................................................................................... 4
6. ENVIRONMENTAL PROTECTION:............................................................................................ 4
7. HOW SUPPLIED:....................................................................................................................... 4
7.1. Accessories: ....................................................................................................................... 4
7.2. Single Use Devices –See table below for other devices required for Tumor Ablation........... 4
8. INSTRUCTIONS FOR USE: ....................................................................................................... 5
8.1. Set-Up ..................................................................................Error! Bookmark not defined.
8.2. MetaSTAR RF Generator Nomenclature...............................Error! Bookmark not defined.
8.3. Initiation.............................................................................................................................. 7
8.4. Setting Ablation Cycle Time ................................................................................................ 7
8.5. Operation............................................................................................................................ 7
9. PREVENTIVE MAINTENANCE, TROUBLESHOOTING AND REPAIR:....................................... 9
9.1. Adjusting Volume................................................................................................................ 9
9.2. Maintaining Device Effectiveness........................................................................................ 9
9.3. Cleaning the MetaSTAR RF Generator..................................Error! Bookmark not defined.
10. CUSTOMER SERVICE AND TECHNICAL SUPPORT, WARRANTY: ......................................... 9
10.1. Customer Service................................................................................................................ 9
10.2. Limited Warranty................................................................................................................. 9
11. DISTRIBUTORS / AUTHORIZED REPRESENTATIVES:.......................................................... 11
11.1. North America.......................................................................Error! Bookmark not defined.
11.2. Europe ................................................................................Error! Bookmark not defined.
APPENDIX A PRODUCT SPECIFICATIONS..................................................................................... 11
APPENDIX B ELECTROMAGNETIC ENVIRONMENT GUIDANCE.................................................... 12
APPENDIX C ABBREVIATIONS ........................................................................................................ 12
APPENDIX D CONFORMANCE TO STANDARDS............................................................................. 13
APPENDIX E COMPATIBLE POWER CORDS .................................................................................. 13
APPENDIX F TONES ........................................................................................................................ 13
APPENDIX G INDICATORS / ERROR CODES AND TROUBLESHOOTING...................................... 14

403321001_001 2016-08-31
MetaSTAR RF Generator User Manual
Catalog No. 3195 (English) (EN)
CAUTION: Federal law (US) restrictsthis device to sale by or onthe order of a physician.
1. BRIEF DEVICE DESCRIPTION:
The MetaSTAR RF Generator is a microprocessor controlled low power radiofrequency (RF) generator used for
percutaneous delivery of bi-polar radiofrequency energy intended to heat the target tissue of the SpineSTAR™
Ablation Instrument.
2. INDICATIONS FOR USE:
The MetaSTAR RF Generator is indicated for palliative treatment in spinal procedures by ablation of metastatic
malignant lesions in a vertebral body.
3. CONTRAINDICATIONS:
In some cases the system may interfere with normal functions of some types of implanted pacemakers. The
MetaSTAR RF Generator is contraindicated for patients who have pacemakers or other electrical implants. Please
refer to the SpineSTAR Ablation Instrument instructions for use for a more comprehensive list of contraindications
regarding specific procedures.
4. WARNINGS:
Warning: Do not ablate in painful osteoporotic vertebra without tumor.
Warning: Do not use this device in patients without metastatic malignant lesions in a vertebral body.
Warning: Do not use this device in patients with multiple myeloma, solitary plasma cytoma, or primary malignant
lesions in the index vertebra.
Warning: The device is designed for tumor ablation; follow the IFU for the applicable device(s) used during the
procedure.
Warning: Hazardous electrical output. This equipment is for use only by qualified medical personnel trained in the
use of the MetaSTAR RF Generator.
Warning: Do not operate the unit in close proximity to volatile solvents such as methanol or alcohol, or in the
presence of flammable anesthetics, as explosion may occur.
Warning: Do not operate unit in moist environment, as a shock hazard may exist. If liquids have entered the unit,
the MetaSTAR RF Generator must be returned to the manufacturer for testing prior to use.
Warning: Interference produced by the operation of high-frequency equipment may adversely affect the operation
of other electronic medical equipment such as monitors, imaging systems.
Warning: Failure of the equipment could result in an unintended increase of output power.
Warning: Use of accessories and cables, other than those specified may affect system performance, resulting in
increased emissions or decreased immunity of the system.
Warning: Servicing otherthanreplacingthefuse should be performed bythe manufacturer.
Warning: Use only hospital grade power cord.
Warning: In case of Generatorfailure switch off/unplug thepower cord.
Warning: Ablation procedures must be performed under fluoroscopic image guidance. Do not perform ablation
without imaging asit may resultin severe injurytopatient.
Warning: Do not remove data portcover or connectany equipment to the dataport.
Warning: To avoid risk of electric shock, this equipment must only be connected to a supply mains with protective
earth.
403321001_001 2016-08-31
5. PRECAUTIONS:
Caution: Removing screws and opening of this device may invalidate the warranty.
Caution: ”.
Caution:It is important to read these instructions for use and these precautions prior to device operation.
Caution: Replace fuses only with the same type and rating: 5 x 20 mm 3.15A/250V(2 each).
Caution: Do not sterilize the MetaSTAR RF Generator. Sterilization may damage the unit.
Caution:Reconditioning, refurbishing, repair, modification, of the device to enable further use is expressly
prohibited as it may result in loss of function and/or patient injury.
Caution: Do not block or restrict the openings on the bottom and back of the MetaSTAR RF Generator, as they
provide the required airflow for cooling.
Caution: If electromagnetic interference with other equipment is suspected, reorient the device or remove
possible sources of interference (e.g., cellular phones, radios, etc.) from the room.
Caution: Any monitoring electrodes recommended to be placed as far as possible from the SpineSTAR Ablation
Instrument when high frequency surgical equipment and physiological monitoring equipment are used
simultaneously on the same patient. Monitoring systems incorporating high frequency current-limiting
devices are recommended for use.
Caution: Needle monitoring electrodes are not recommended.
Caution: Patient should not come into contact with earthed metal parts, the use of antistatic sheeting is
recommended.
Caution: Cables to the surgical electrodes are recommended to be positioned such that contact with patient or
other leads is avoided.
Caution: Do not continue to deliver RF for more than 30 seconds if the impedance bar is morethan 75% filled
andtemperatureis not increasing.
The lightning flash with arrowhead symbol, within an equilateral triangle, is intended to alert the
user to the presence of un-insulated "dangerous voltage" within the products enclosure that may
be of sufficient magnitude to constitute a risk of electric shock to persons.
The symbol with an open book with the letter “i” is intended to alert the user to the presence of
important operating and maintenance (servicing) instructions in the literature accompanying the
unit.
The symbol with a person reading an open book is intended to alert the user to read and
understand the operator’s manual prior to use. Failure to follow instructions could result in death
or serious injury.
6. ENVIRONMENTAL PROTECTION:
Follow local governing ordinances and hospital practice regarding the disposal of the SpineSTAR Ablation Instrument
(see SpineSTAR Ablation Instrument IFU)
7. HOW SUPPLIED:
The MetaSTAR RF Generator is supplied in a semi-ready-to-use state. The units’ box contains the MetaSTAR RF
Generator, and a detached power cord. All other devices are available separately.
7.1. Accessories:
7.1.1. Hand Switch Cable
7.1.2. AE Cable
7.2. Single Use Devices –See below for other devices required for Tumor Ablation.
SpineSTAR Ablation Instrument*
* Applied Part, VPPMax: Same as Output. See Appendix A, I. Specifications.

403321001_001 2016-08-31
8. DEVICE PREPARATION:
8.1. Cleaning the MetaSTAR RF Generator
Clean the MetaSTAR RF Generator prior to use with a damp cloth and water, isopropyl alcohol, 1.5%
hydrogen peroxide, or a mild bleach solution after each use. Prolonged exposure to any corrosive
solvents or disinfectants should be avoided.
8.2. Device Set Up
Examine the unit for damages prior to use. DO NOT use a damaged device.
Verify that the unit is fully operational prior to use.
Connect the power cord to the Power Socket (8.2) on the back of the MetaSTAR RF Generator and to an
electrical outlet. The power requirements for the MetaSTAR RF Generator are located on the label on the back
of the unit.
Turn on the MetaSTAR RF Generator power and see if it passes self-test prior to use. Examine the SpineSTAR
Ablation Instrument, AE Cable, and Hand Switch Cable prior to use. DO NOT use a damaged device.
Make sure that at least one disposable SpineSTAR Ablation Instrument, Hand Switch Cable, and AE Cable
are available as backup, prior to use.
8.3. MetaSTAR RF Generator Nomenclature & Symbols
“ON” (power)
TypeBF Applied Part
VolumeControl
“OFF” (power)
Warning, electricity
Up Arrow
Non-ionizing
electromagnetic
radiation
Reset Cycle
Down Arrow
Equipotentiality
Reset All
DataOutput
ProtectiveEarth
Termination
RF Off
RF Iconondisplay
Fuse
Confirm
UL Classified Medical
Equipment
with respect to electrical
shock, fire and mechanical
hazards only in accordance
with ANSI/AAMI ES 60601-1
(2005, 3rd), IEC60601-1,
CAN/CSA C22.2 No. 60601-
1 (2008), IEC 60601-2-2
(2009)
Caution
(see Instruction for
Use)
Use By Date
Keep dry
Consult instructions for
use
Do not use if package is
damaged
Consult accompanying
documents for system
disposal
Readand understand
operator’s manual prior
to use! Failuretofollow
instructionscould result
indeath or seriousinjury.
Keep away from
sunlight
Sterilized using
Irradiation
Serial Number
Date of Manufacture
Temperature limit
Catalog Number
Manufacturer
Fragile, handle with care
Do no re-use
Authorized
Representative in the
European Community

403321001_001 2016-08-31
Indicators and Sockets
1. Graphical Display
2. FAULT RED LED
3. WARNING YELLOW LED
4. RF ON BLUE LED
5. POWER ON GREEN LED
6. Soft Key Button 1
7. Soft Key Button 2
8. Soft Key Button 3
9. Soft Key Button 4
10. Hand Switch Cable Socket
11. AE Cable Socket
12. Fuse Drawer
13. ON/OFF Main Switch
14. Power Socket Equipotential
15. Ground Lug
16. Speaker Volume Knob
17. Data Output Port

403321001_001 2016-08-31
9. INSTRUCTIONS FOR USE:
9.1. Initiation
Once the device Preparation (8.0) is complete and power is turned on using the ON/OFF Main Switch
at the back of the MetaSTAR RF Generator, the system performs a self-check. All LEDs and pixels
on the graphics display will be on and the speaker will sound for a few seconds upon initiation (Power-
On Tone). In case an error is detected during Self-Test the Fault or Warning LED will turn on and the
Fault or Warning Tone will sound.
NOTE: The Hand Switch Cable can be inserted in the Hand Switch Cable Socket prior to powering
up the MetaSTAR RF Generator.
After self-check is performed, the software versions are displayed.
9.2. Setting Ablation Cycle Time
Upon completion of the self-check the generator will go to the set Ablation Cycle Time Screen. In
the Ablation Cycle Time Screen pressing the Up and Down Arrow Buttons will increase or decrease
the RF Cycle time. Once the desired time is selected, pressing the Confirm Button will enter the time
and advance to the Main Procedure Screen. During operation (below), the user can re-set the
Ablation Cycle Time by pressing and holding the Reset Cycle Button for 2seconds.
9.3. Operation
SpineSTAR Ablation Instrument and AE Cable
“Connect Device” will flash until the SpineSTAR Ablation Instrument is connected to the MetaSTAR
RF Generator. The SpineSTAR Ablation Instrument should be connected to the AE Cable socket on
the MetaSTAR RF Generator using the AE Cable (inspect the AE Cable for damage). If the
SpineSTAR Ablation Instrument is connected properly, Distal and Proximal temperature readings will
display.
Hand Switch Cable
The Hand Switch Cable should be inspected and inserted into the Hand Switch Cable socket on the
MetaSTAR RF Generator.
The MetaSTAR RF Generator will display the following information:
1. RF Cycle: Displayed at the top left portion of the Generator Display in minutes:seconds. (00:00)
2. Cycles: Displayed at the top center portion of the Generator Display in number of cycles (00)
performed.
3. RF Total: Displayed at the top right portion of the Generator display in minutes:seconds (00:00) format.
This value represents the cumulative time RF power has been delivered.
4. Distal TC: Displayed in (ºC): Temperature of the Distal Thermocouple of the SpineSTAR Ablation
Instrument.
5. Proximal TC: Displayed in (ºC): Temperature of the Proximal of the SpineSTAR Ablation Instrument.
6. Ω (Impedance): Displayed as a box filling from bottom to top on bottom left of Generator’s display.
7. Power: Displayed in (Watts): Ablation RF output power of the MetaSTAR RF Generator.
RF Power Level
This generator has four power settings, 3w, 5w, 7.5w and 10w.
The selected output power should be as low as possible for the intended purpose. Certain devices
or accessories may present SAFETY HAZARDS (See WARNINGS and PRECAUTIONS above). Set
the power level to 5W and modify during procedure as required to create thermal distribution for the
major and minor diameter as displayed in Figure 1.

403321001_001 2016-08-31
Ablation Zone Dimensions
The below table depicts the ablation zone sizes for each device:
Ablation Zone Dimensions
5│10 SpineSTAR Ablation Instrument
(Note: All lengths have a tolerance of ±1mm)
10│15 SpineSTAR Ablation Instrument
(Note: All lengths have a tolerance of ±1mm)
Figure 1: Ablation Zone
Caution: Excessive ablation may create heat within the spine and adjacent neural tissue.
STAR Tumor Ablation Procedure
By pressing the BLUE button on the hand switch, the user can start ablation when the SpineSTAR
Ablation Instrument is properly placed:
1. Both thermocouple temperatures are displayed in amber (≥ 30°C). Note, temperatures
are displayed in blue when temperature is <30°C.
2. Electrode is extended to the desired location (impedance is within range).
During RF delivery, a RF Enable tone will sound. If the temperature for proximal TC is greater than
45°C, High Temperature tone will replace the normal RF tone.
The user can stop RF delivery by pressing the Blue button on the hand switch again.
RF will also automatically turn off if any of the following criteria are met:
1. RF Cycle Time reaches 00:00.
2. Maximum tissue impedance (1000Ω) is achieved.
10mm TC
5mm TC
Width
Length
Distal
Proximal
15mm TC
10mm TC
Length
Width
Distal
Proximal

403321001_001 2016-08-31
3. Proximal Thermocouple (TC) on the SpineSTAR Ablation Instrument reaches 50ºC.
The RF Cycle Time can be reset to the pre-set value by pressing and releasing the Reset Cycle
Button.
The RF Total Time, number of Cycles, and RF Cycle Time can be reset together by pressing and
holding the Reset All button for 2 seconds.
Completion of Procedure
Turn off MetaSTAR RF Generator using the power button. Unplug the controller from the electrical
outlet (Mains) and remove the power cord from the back of the controller.
10. PREVENTIVE MAINTENANCE, TROUBLESHOOTING AND REPAIR:
10.1. Adjusting Volume
The MetaSTAR RF Generator has an adjustable volume control (Section 8.2 item 16) on the back of
the unit. Twisting the adjustor clockwise will increase the volume.
10.2. Maintaining Device Effectiveness
In the event of a blown fuse, only 5x20mm 3.15A/250VAC Type “T” (slow blow) fuses should be used
as replacements. Turn power off and disconnect the power cord from the electrical outlet. Remove
fuse holder by opening the Power Entry Module’s Fuse Drawer (Section 8.2 item 12) on the back of
the MetaSTAR RF Generator. Replace both fuses.
Besides the fuses there are no other user serviceable parts. No servicing or preventive maintenance
is required for this device. For servicing, replacement or repair, return cleaned unit (9.3) to
manufacturer.
11. CUSTOMER SERVICE AND TECHNICAL SUPPORT, WARRANTY:
11.1. Customer Service
Contact MERIT’s Customer Service for any customer or technical support.
Call +1-800-356-3748 or e-mail us at customerservi[email protected]om.
11.2. Limited Warranty
Merit Medical Systems, Inc. (“MERIT”) warrants to the original purchaser of the MetaSTAR RF
Generator (“MetaSTAR RF Generator”) that the MetaSTAR RF Generator will be free of defects in
material and workmanship for a period of one year after the date of purchase when used as intended
under normal operating conditions and in conformance with their instructions for use and
maintenance instructions. The sole obligation of MERIT under this warranty shall be limited to the
replacement of the MetaSTAR RF Generator or equivalent models, at no charge to the original
purchaser and at the option of MERIT, during the warranty period but only if examination reveals to
the satisfaction of MERIT that the MetaSTAR RF Generator contains a defect in material or
workmanship.
This warranty is made in lieu of all other warranties, expressed, implied or statutory, including, but
not limited to the implied warranties of merchantability and fitness for a particular purpose and all
other obligations and liabilities on the part of MERIT. MERIT neither assumes nor authorizes any
other person to assume for it any other liability in connection with the sale of the MetaSTAR RF
Generator. This warranty shall not apply to MetaSTAR RF Generator or any other part thereof that
has been subject to accident, negligence, alteration, abuse, or misuse. MERIT makes no warranty
whatsoever with regard to accessories or parts used in conjunction with the MetaSTAR RF Generator
and not supplied and manufactured by MERIT.
The term “original purchaser,” as used in the warranty means that person or organization and its
employees, if applicable, to whom the MetaSTAR RF Generator was sold by MERIT. This warranty
may not be assigned or transferred in any manner.
In order to make a warranty claim, the original customer must obtain a Return Good Authorization
Number (“RGA Number”) and return instructions from MERIT’s Customer Service Department or
authorized representative. The allegedly defective MetaSTAR RF Generator must be returned,
cleaned, repackaged, clearly marked with the RGA number on all packaging, and adequately insured,
to the following address: Merit Medical Systems, Inc., 1600 West Merit Parkway, South Jordan, Utah
84095 U.S.A. A written statement must also be included that reasonably describes the defect(s) with
the MetaSTAR RF Generator and indicates the appropriate return address to ship the replaced
MetaSTAR RF Generator, as well as a copy of a receipt showing proof of purchase.

403321001_001 2016-08-31
Manufactured By:
Merit Medical Systems, Inc.
1600 West Merit Parkway
South Jordan, Utah 84095 U.S.A.
1-801-253-1600
U.S.A. Customer Service 1-800-
356-3748
Authorized Representative:
Merit Medical Ireland Ltd.
Parkmore Business Park West
Galway, Ireland
+31 43 358 82 22

403321001_001 2016-08-31
APPENDIX A PRODUCT SPECIFICATIONS
I. Specifications
Mode of Operation:............................................................................... Continuous
Input: ........................................................................100-240VAC, 50-60Hz, 120VA
Dimensions:............................................. 6 ”(H) x 11”(W) x 18”(D) (15 x 28 x 46cm)
Weight: ................................................................................................ 6.6 lbs (3kg)
Output: ............................................................10WMAX, 480kHz, 140VPPMax 250Ω load
Fuses: ......................................5x20mm 3.15A/250VAC Type “T” (time-lag) (Qty. 2)
Weight and dimensions indicated are approximate. Specifications are subject to change without
notice.
II. Protection
Class 1, Type BF, continuous operation; Enclosure IP 21
III. Operating Conditions
Temperature: ............................................................. 59°F to 104°F (15°C to 40°C)
Relative Humidity:...................................................... 30% to 75% non-condensing
Atmospheric Pressure:...................................... 878 to 1082cmH2O (86 to 106kPa)
Whenever the temperature goes above 50°C or below 10°C and the MetaSTAR RF Generator
is in Standby or RF Enabled mode, it goes into a thermal shut down.
IV. Transport and Storage Requirements
Temperature: ..............................................................0°F to 104°F (-18°C to 40°C)
Relative Humidity: ......................................................10% to 90% non-condensing
V. Tumor Ablation Mode Output Power
0
2
4
6
8
10
12
0100 200 300 400 500 600 700 800 900 1000
Output Power - Watts
Impedance - Ohms
Output Power vs Load Impedance
3w
5w
7.5w
10w

403321001_001 2016-08-31
APPENDIX B ELECTROMAGNETIC ENVIRONMENT GUIDANCE
APPENDIX C ABBREVIATIONS
LED Light Emitting Diode
RF Radio Frequency
Electromagnetic Environmental Guidance
Electromagnetic Emissions
The MetaSTAR RF Generator and SpineSTAR Ablation Instrument are intended for use in the
electromagnetic environment specified below. The customer or the user of the System should assure that
it is used in such an environment.
Emissions test
Compliance
Environmental guidance
RF emissions CISPR 11
Class A
Harmonic emissions IEC 61000-3-2
Class A
Voltage fluctuations/ flicker emissions IEC 61000-
3-3
AC Input
The System does not require interconnection with other equipment.
Portable and mobile RF communications equipment can affect operation of the System.
The System intentionally radiates RF in normal operation.
Electromagnetic Immunity
The MetaSTAR RF Generator and SpineSTAR Ablation Instrument are intended for use in the
electromagnetic environment specified below. The customer or the user of the System should assure that
it is used in such an environment.
Immunity test
IEC 60601 test level
Compliance
level
Environmental
guidance
Electrostatic discharge (ESD)
IEC 61000-4-2
Contact:
±2kV, ±4kV±6kV
Air:
±2kV, ±4kV ,±6kV ±8 kV
Per test level
Radiated RF Electromagnetic
Fields IEC 61000-4-3
80 to 1000Mhz 3V/m
AM 80%
3 sec dwell
Per test level
Electrical fast transient/burst
IEC 61000-4-4
Test at AC Port for both
230Vac/50hz and
120Vac/60hz input voltages
Per test level
Surge IEC 61000-4-5
Test at AC Port for both
230Vac/50hz and
120Vac/60hz input voltages
Per test level
Conducted disturbances,
Induced by RF Fields
IEC 61000 -4 -6
0.15Mhz to80Mhz 3V/m AM
3 sec dwell
Per test level.
Voltage dips, short
interruptions and voltage
variations on power supply
input lines IEC 61000-4-11 UT
= 230 Vac
Test at AC Port for both
230Vac/50hz and 120Vac/60
hz input
Power frequency (50 Hz)
magnetic field IEC 61000-4-8
Test at AC Port for both
230Vac/50hz and
120Vac/60hz input 3A/m
Per test level

403321001_001 2016-08-31
APPENDIX D CONFORMANCE TO STANDARDS
The MetaSTAR RF Generator conforms to the following International Standards:
EN 60601-1 Medical Electrical Equipment. Part 1: General Requirements for
Basic Safety and Essential Performance
AAMI ES 60601-1 Medical Electrical Equipment. Part 1: General Requirements for
Basic Safety and Essential Performance
CSA C22.2 No. 60601-1 Medical Electrical Equipment. Part 1: General Requirements for
Basic Safety and Essential Performance
IEC 60601-1-2 Medical Electrical Equipment, Part 1-2: Collateral Standard:
Electromagnetic Compatibility
IEC 60601-2-2 Medical Electrical Equipment, Part 2-2: Essential Performance
Particular Requirements for the Basic Safety and Essential Performance
of High Frequency Surgical Equipment and high frequency surgical
accessories
ANSI/AAMI/ISO 15223 Graphical Symbols for Use on Equipment
EN 60529 Degrees of Protection Provide by Enclosures (IP Code)
ANSI/AAMI ES-1 Safe Current Limits for Electro medical Apparatus
ASTM D4169 Standard Practice for Performance Testing of Shipping
Containers & Systems
EN 55011 (CISPR 11)Limits and Methods of Measurement of Electromagnetic Disturbance
Characteristics of Industrial, Scientific, and Medical (ISM) RF
Equipment
MDD 93/42/EEC Medical Device Directive (Council Directive Concerning Medical
Devices)
APPENDIX E COMPATIBLE POWER CORDS
Continental Europe, 2.0m, 10A, Inter Power PN 86231000
Argentina, 2.5m, 10A, Inter Power PN 86270010
Brazil, 2.5m, 10A, Inter Power PN 86286110
China, 2.5m, 10A, Inter Power PN 86517040
India / South Africa, 2.5m, 10A, Inter Power PN 86265010
Israel, 2.5m, 10A, Inter Power PN 86275010
Italy, 2.5m, 10A, Inter Power PN 86394000
Japan, 2.3m, 7A, Inter Power PN 86589000
Switzerland, 2.5m,10A, Inter Power PN 86285010
United Kingdom / Ireland, 2.5m,10A, Inter Power PN 86240060
Hospital-Grade
Australia/New Zealand, 2.5m, 10A, Inter Power PN 86210140
Denmark, 2.5m, 10A: Inter Power PN 86280810
North America, 3.0m, 10A Inter Power 86610710 rev B
APPENDIX F TONES
Power On –Speaker: 250Hz for 75ms, 300Hz for 100ms, 400Hz for 100ms, 500Hz for 80ms,
530Hz for 100ms, 600Hz for 100ms, 750Hz for 100ms, 900Hz for 100ms, 100ms silence, 1000Hz
110ms five times within 5 seconds, 1000Hz for 500ms tone once at power up.
RF Enabled –Speaker: 300Hz for 200ms –once every two seconds when RF energy is
delivered in Ablation Mode.
Fault - High Priority tone.
Warning - Medium Priority tone.
Button Click - The tone pattern shall be the following: 1000Hz for 20ms.
Temperature High –Speaker: 600Hz for 200ms –once every two seconds when RF energy is
delivered in Ablation Mode and the corresponding TC reading is above 45 °C.

403321001_001 2016-08-31
APPENDIX G INDICATORS / ERROR CODES AND TROUBLESHOOTING
In event of Hand Switch Cable failure – e.g. Hand Switch is stuck – remove Hand Switch Cable
connector from MetaSTAR RF Generator.
If stuck in “ON” position turn off MetaSTAR RF Generator.
In the event of a thermal shut down (ambient temperature is above 50°C or below 10°C) the
MetaSTAR RF Generator stays in Warning mode until temperature returns to normal operating
conditions. Resolve by turning off and on when temperature is between 10°C-50°C.
This equipment has been tested and found to comply with the limits for medical devices to the IEC
60601-1-2. These limits are designed to provide reasonable protection against harmful interference
in a typical medical installation. This equipment generates; uses and can radiate radiofrequency
energy and, if not installed and used in accordance with the instructions, may cause harmful
interference to other devices in the vicinity. However, there is no guarantee that interference will not
occur in a particular installation. If this equipment does cause harmful interference to other devices,
which can be determined by turning the equipment off and on, the user is encouraged to try to
correct the interference by one or more of the following measures:
Reorient or relocate the receiving device.
Increase the separation between the equipment.
Connect the MetaSTAR RF Generator to an electrical outlet on a circuit different from the
affected equipment.
In case of unsuccessful Self-Test FAULT LED or WARNING LED will be on, continuous alarm
Fault or Warning Tone –Requires System Restart.
In case of Error State, the unit will remain in that Fault or Warning condition until the unit is
switched off using the MAIN ON/OFF POWER SWITCH.
In the event the MetaSTAR RF Generator does not recognize the SpineSTAR Ablation
Instrument it will flash “Connect Device.”
Warnings and Fault Messages
Warning Messages displayed on MetaSTAR RF Generator
All the Warning messages follow the basic form below:
Example of Warning message (in Yellow outlined triangle and text):
Fault Messages displayed on MetaSTAR RF Generator
All the Fault messages follow the basic form below:
Example of Fault message (in Red outlined triangle and text):
403321001_001 2016-08-31
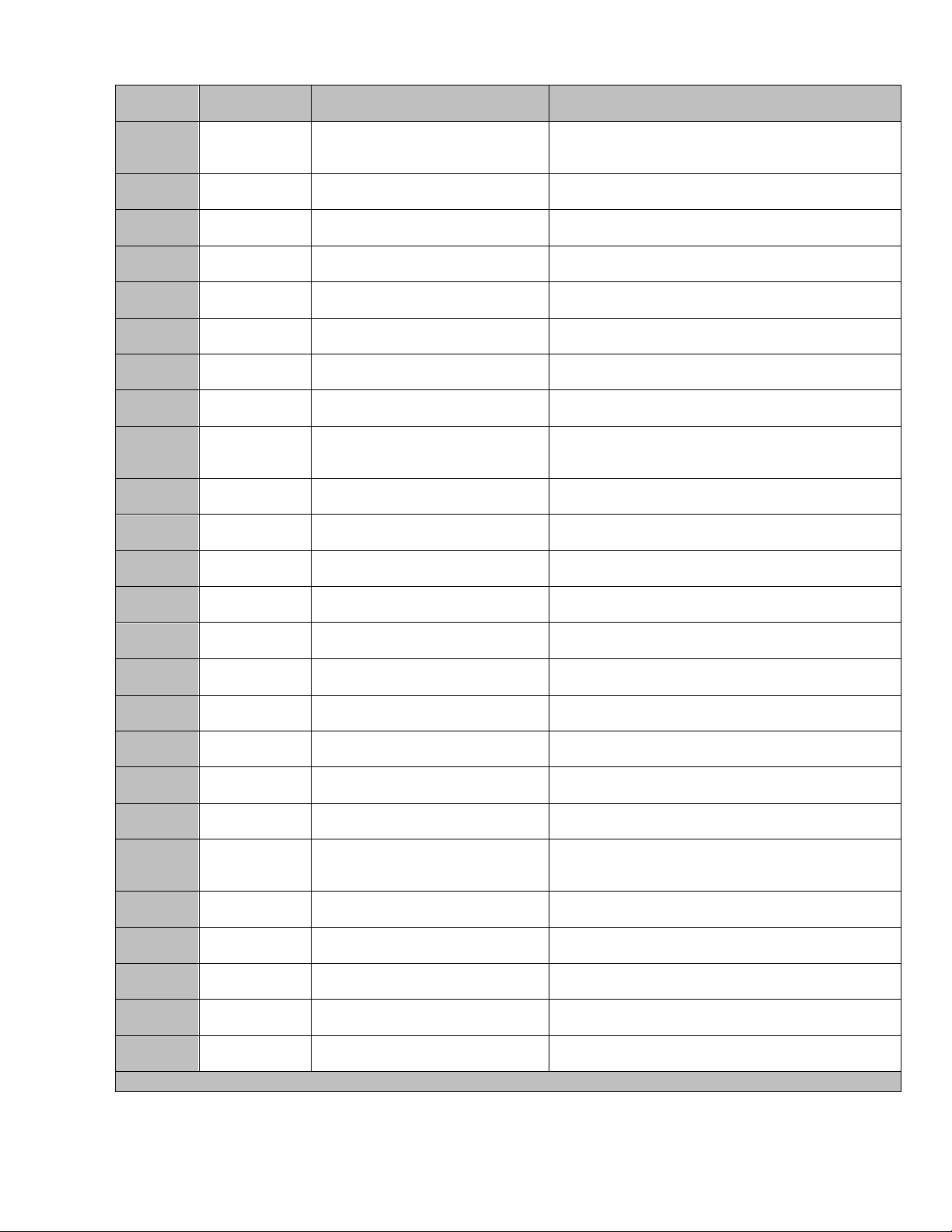
Provided below is a list of Warning and Fault messages, listed in ascending order.
Message
Number
Fault (F) /
Warning (W)
What Fault Message Means
User Actions
28*
W
Load impedance too high, RF
circuits getting hot.
Turn Power off for 10 minutes to let generator cool
down. Turn Power back on. If error persists,
contact MERIT Customer Service.
29
F
RF power level too high during
treatment
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
30
W
Impedance Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
31
F
RF Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
32
F
24V Supply Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
33
F
12V Supply Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
34
F
-12V Supply Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
35
F
5V Supply Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
36*
W
Temperature Power On Self Test
Turn Power off until temperature is between 50°C-
10°C. Turn Power back on. If error persists,
contact MERIT Customer Service.
37
F
SPI Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact Customer Service.
38
F
Cold junction compensator
Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
39
F
Watchdog Fault
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
41- 53
W
RAM Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
60 - 66
W
Display Graphic
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
70
F
EEPROM Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
71
F
DAC or ACD Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
72
W
Display Initialization Power On Self
Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
73
F
CPU Configuration Power On
Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
74
F
CRC Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
75*
W
Stuck or Shorted Panel Buttons or
Hand Switch Buttons
Turn Power off. Push all buttons once. Turn
Power back on. If error persists, contact MERIT
Customer Service.
80 - 86
W
Display Graphic
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
95
F
“Fault and Warning Alarm”
Power On Self Test
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
96
W
Tone Generator Software Version
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
97
F
“Fault and Warning Alarm”
Watch Dog
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
98
F
“Fault and Warning Alarm”
Processor or Program
Turn Power off. Turn Power back on. If error
persists, contact MERIT Customer Service.
* Recoverable-system error, follow instructions listed in User Actions column.
Table of contents
Popular Portable Generator manuals by other brands

Briggs & Stratton
Briggs & Stratton 40277 Illustrated parts list

Telair
Telair Energy 4010G Manual for installation and user manual

R&S
R&S SMCVB-K164 user manual

Generac Power Systems
Generac Power Systems GP Series owner's manual

Rem
Rem SPU48 quick start guide

IFM
IFM ProcessLine AC295 Series operating instructions











