Neuropace 5106 User manual

Remote Monitor
Manual
Model 5106
NeuroPace® Patient Materials
THE RNS® SYSTEM
This manual includes information for the use of the NeuroPace® Remote
Monitor and is a supplement to your RNS® System Patient manual. This
manual is not meant to take the place of advice from your doctor. For a
complete discussion of indications for use, contraindications, warnings,
cautions, and potential side eects, talk to your doctor.
© 2023 NeuroPace, Inc.
DN 1019078 Rev. 6
Rev. Date: 2023-08

Remote Monitor Manual Model 5106
Contents
2 FCC Information
2 Electromagnetic Emissions
and Immunity and
Wireless Technology
3 Symbols
4 Safety Information
Warnings…4
Cautions…7
Cybersecurity, Device Security, and
Patient Information…10
12 About
Indications for Use...13
Purpose of Your Remote Monitor...13
14 Parts
Carrying Case...15
The Remote Monitor Software On Tablet
Computer...15
Wand...15
Power Cord...15
16 Setup
Find a Good Place to Setup...17
Charge the Remote Monitor...17
Turn on the Remote Monitor...19
20 Basics
Tablet Touchscreen and Buttons...21
Home Screen...21
Connect to Wi-Fi...22
Wi-Fi Button on the Home Screen...24
Confirm Remote Monitor Setup...24
Turn O The Remote Monitor...25
26 Get Data
30 Wand
Wand Signal Basics...31
Understanding Signal Level and
Quality...32
Wand Too Far Away or Wand
Disconnected...32
34 Update Center
Update Types...35
Update Options...35
Update Waiting...36
Update Process and Power
Requirements...37
38 Travel
Airport Security...39
Traveling...39
40 Care and Maintenance
Care and Maintenance...41
Return / Disposal Of Tablet Computer And
Wand...41
42 Troubleshooting
Remote Monitor Problems...43
Wand Problems...44
Data Collection Problems...45
Problems Uploading Data...46
58 If You Need Help

Remote Monitor Manual Model 5106
Electromagnetic Emissions and
Immunity and Wireless Technology
Medical Electrical Equipment needs special
precautions regarding EMI and the following
precautions should be taken before use.
The remote monitor may cause radio
interference or may disrupt the operation of
nearby equipment. The remote monitor may
be interfered with by other equipment, even
if that other equipment complies with CISPR
emission requirements. It may be necessary to
take mitigation measures, such as re-orienting
or relocating the remote monitor or shielding its
location.
The remote monitor should not be used
adjacent to or stacked with other equipment.
If adjacent or stacked use is necessary, the
remote monitor should be observed to verify
normal operation in the configuration in which it
will be used.
Portable and mobile RF communications
equipment can aect the remote monitor. Refer
to Electromagnetic Emissions and Immunity
on page 48 for more information.
FCC Information
The following is communications regulation
information on the neurostimulator models
RNS-300M and RNS-320, and wand model
W-02.
• Neurostimulator model RNS-300M FCC ID:
WBWRF300
• Neurostimulator model RNS-320 FCC ID:
WBWRF320
• Wand FCC ID: WBW5200 or WBW902
These components comply with Part 15 of
the FCC Rules. Operation is subject to the
following two conditions: (1) These devices may
not cause harmful interference, and (2) these
devices must accept any interference received,
including interference that may cause undesired
operation.
Important:
Changes or modifications to these components
not expressly approved by NeuroPace, Inc.
could void the FCC Certification, and negate
your authority to operate them.
This equipment complies with FCC radiation
exposure limits set forth for an uncontrolled
environment. This transmitter must not be co-
located or operating in conjunction with any
other antenna or transmitter.
2
FRONT MATTER / EMI

Remote Monitor Manual Model 5106
Proposition 65, a State of California voter initiative, requires the following notice:
WARNING: This product can expose you to chemicals including ethylene oxide, which is
known to the State of California to cause cancer and birth defects or other reproductive
harm. For more information go to www.P65Warnings.ca.gov.
Symbols
SYMBOLS
Explanation of symbols on product or package labeling
Symbol Explanation Symbol Explanation
Caution
Use
Temperature limits
during use
MR MRI Unsafe
MR
MR Conditional
Storage
Temperature limits
during storage or
transport
Prescription Only
SN
Serial Number Type BF applied part
5 V, 0.5 A
Direct current (DC) 5 V
(volts), 0.5 A (amperes)
Class II electrical
protection
IP22
Ingress protection ratings: Level 2 for solid objects, which means testing
confirms the device enclosure prevents ingress (entry) of items greater
than 12.5 mm (~1/2 inch), such as fingers or similar objects; Level 2 for
liquids, which means testing confirms vertically dripping water shall have
no harmful eect when the enclosure is tilted at an angle up to 15° from
its normal position.
Note: Images in this manual are representative and may vary in detail from what a particular
user experiences.
3
SYMBOLS

Remote Monitor Manual Model 5106
Warnings
WARNING: Medical Procedures
DO NOT have any of the following procedures
before making sure the person administering
the procedure knows that you have the RNS®
System implanted and they have consulted with
the doctor who is monitoring your use of the
RNS® System:
• Computerized Tomography (CT or CAT)
scans.
• Radiation therapy (such as cobalt 60 or
gamma radiation to treat cancer).
• Lithotripsy (shock waves to break up hard
masses, such as kidney stones).
• Electrolysis (electrical current to remove
unwanted hair).
The energy used in these procedures may
damage the RNS® System. This could result
in stimulation not being delivered, additional
surgery to remove or replace parts of the RNS®
System, serious injury, or death.
Lithotripsy and Electrolysis should not be
performed on the head or neck.
In addition, Computerized Tomography (CT or
CAT) scans should be performed only under the
following conditions:
• The neurostimulator should be turned o
prior to the procedure if possible. This
should be done by your doctor or someone
who is authorized to adjust the settings
using the programmer.
• The scan should be taken at the lowest
X-ray beam level possible.
• Avoid directing the beam at or near the
implant site for more than a few seconds.
• Emergency services need to be available
in the event you have a serious side eect.
This is especially important if the scan area
includes the implant site.
• The neurostimulator should be turned back
on after the procedure.
WARNING: MRI Safety Information
MR
RNS® Neurostimulator model
RNS-320: You must consult your
doctor to determine whether an
MRI scan is possible for you. The
RNS® System (with RNS
Neurostimulator model RNS-320) is MR
Conditional, which means that an MRI scan may
be safely performed under specific conditions.
It may also be possible to have an MRI scan if
your neurostimulator has been explanted. If
your doctor determines it is possible for you to
have an MRI scan, he or she must make sure
that the conditions for a safe MRI scan are
followed. Those conditions include putting your
neurostimulator in MRI Mode, which uses more
battery and may aect battery life. While in MRI
Mode, the neurostimulator is not detecting or
delivering therapy to the patient. Therefore, you
should work with your doctor to minimize the
time your neurostimulator is in MRI Mode.
MR
RNS® Neurostimulator model
RNS-300M: DO NOT have a
Magnetic Resonance Imaging (MRI)
scan if you have an RNS® System
with an RNS Neurostimulator model
RNS-300M implanted. The RNS Neurostimulator
model RNS-300M is MR Unsafe. Having an MRI
scan with an RNS Neurostimulator model
RNS-300M implanted may result in serious
injury or possible death.
MR
RNS® System External
Components: All external
components such as the magnet,
wand and remote monitor are MR
Unsafe and can pose a projectile
hazard, and therefore, must be kept out of the
MRI scanner room.
Contact your doctor as soon as possible if you
have questions or suspect your RNS® System
is not working properly after any medical
procedure.
Safety Information
4
SAFETY INFORMATION
4

Remote Monitor Manual Model 5106
WARNING: Interaction with Implanted Cardiac
Devices
Possible eects of RNS® System interaction
with an implanted cardiac device (such as
pacemakers or defibrillators) include the
following:
• Defibrillation therapy from an implanted
defibrillator may damage the RNS® System.
The electrical pulses from the RNS® System
may interact with the sensing operation
from a cardiac device and could result in an
inappropriate response of the cardiac device
and vice versa.
WARNING: Adverse Tissue Reaction
Allergic reaction to the RNS® System materials
and/or leads implanted is possible.
WARNING: Chronic Tissue Stimulation
The eects of long-term brain stimulation are
not completely known and may present some
risks to the patient.
WARNING: Erosion
Skin erosion (breakdown of skin tissue) may
occur on and/or around the neurostimulator
and/or lead implant site, particularly in the case
of protrusion of the implanted RNS® System
products above the surface of the skull.
WARNING: Lead Migration
The implanted lead(s) may migrate (move)
from their desired implant location. Lead
migration can result in changes in detections
and stimulation eectiveness, and may require
additional surgical procedures to modify the
lead location.
WARNING: Pregnant Women
The safety and eectiveness of the RNS®
System has not been studied in pregnant
women.
WARNING: RNS® System Failure
As with any electronic device, the RNS® System
may malfunction (not work). Potential causes
include battery malfunctions, an electrical
short, open circuits, lead fractures, lead
insulation failures, or damage as a result of head
trauma. These malfunctions are unpredictable,
and may result in too little stimulation or no
stimulation. A lead failure may result in the
lead needing to be removed or repositioned,
which would require surgery. A malfunctioning
neurostimulator may need to be replaced,
which would require surgery. Although the
device is designed to turn o if overstimulation
or excess current occurs, there is a possibility
that product failure could result in brain tissue
damage.
WARNING: Case Damage
If the neurostimulator case is ruptured or
pierced due to outside forces, severe brain
tissue damage could result from exposure to
the battery chemicals.
WARNING: Electrical Shock
To avoid electrical shock (as with any electronic
device such as a tablet computer):
• DO NOT use the wand or tablet when you
are wet.
• DO NOT apply water or liquids directly to
the wand or tablet.
• DO NOT modify the power cord that came
with your remote monitor in any way. If your
remote monitor came with a 3-pronged
plug, connect it to an outlet that accepts
that type of plug.
• DO NOT use the wand or tablet during an
electrical storm.
• DO NOT clean the wand or tablet with
any cleaning liquids or aerosols. Wipe the
outside of the wand and tablet with a clean
cloth, dampened with water and wrung out.
Make sure to disconnect the tablet from the
electrical outlet before cleaning.
5
SAFETY INFORMATION

Remote Monitor Manual Model 5106
• DO NOT use the wand or tablet if you think
they appear to be damaged or are not
working properly.
• DO NOT attempt to modify or repair
the wand or tablet. Contact NeuroPace
Customer Support for assistance.
Not following these instructions may cause an
electrical shock that may result in serious injury
or death, and may damage the wand or tablet.
WARNING: NeuroPace® Equipment Placement
Use of NeuroPace® equipment (for example,
remote monitor or programmer) adjacent to
or stacked with other equipment should be
avoided because it could result in improper
operation. If such use is necessary, the
NeuroPace® equipment and other equipment
should be observed to verify that they are
operating normally.
WARNING: Electromagnetic Interference
(EMI)
Electromagnetic interference is a field of energy
generated by equipment found in the home,
work, medical, or public environments that is
strong enough to interfere with neurostimulator
function. Sources of strong electromagnetic
interference can result in the following eects:
• Serious injury or death - It is possible for
the interference sources to couple enough
energy into a neurostimulator system to
damage brain tissue.
• System damage - resulting in a loss or
change in symptom control and requiring
reoperation.
• Operational changes to the neurostimulator
- causing stimulation to turn on or o,
or resetting or reprogramming the
neurostimulator resulting in a return of
symptoms.
• Unexpected changes in stimulation -
causing a momentary increase in stimulation
which may be felt.
You should exercise caution to avoid devices
which generate a strong electric or magnetic
field. Refer to Electromagnetic Emissions and
Immunity on page 48 for more information.
WARNING: Radio Frequency Identification
(RFID) Interference
RFID scanners can produce signals that appear
as brain activity to the neurostimulator. Such
signals could cause the neurostimulator to
deliver stimulation. Potential sources of RFID
may occur in a health care environment, retail
stores, public libraries, airports and business
environments.
Refer to Electromagnetic Emissions and
Immunity on page 48 for more information.
WARNING: NeuroPace Components
Use of accessories, transducers, and cables
other than those provided by NeuroPace could
result in increased electromagnetic emissions
or decreased electromagnetic immunity of the
RNS® System and result in improper operation.
WARNING: Portable and Mobile Radio
Frequency (RF) Communications Equipment
Portable and mobile RF communications
equipment (including peripherals such as
antenna cables and external antennas) should
be used no closer than 12 inches (30 cm) to
any part of the RNS® System, including cables.
Otherwise, degradation of the performance of
the RNS® System could result.
WARNING: Airport Security and Other
Surveillance Systems
Tell people working with security and theft
systems that you have the RNS® System
implanted and show your medical implant
identification card. Walk through the center of
security screening units without stopping, when
possible, and exit the area of the screening
device as soon as possible. Leave the security
area as soon as practical. Security screening
devices (such as theft detectors, security tag
deactivators, and airport security screening
devices) may be found at retail stores, public
libraries and airports. Such devices use
technology that can cause or temporarily
disrupt stimulation while you are being scanned.
For more information, contact your local airport
security oce or TSA (Transportation Safety
Administration).
6
SAFETY INFORMATION

Remote Monitor Manual Model 5106
WARNING: Wand Placement
DO NOT use (position) the wand over any other
medical device. This includes other implanted
devices such as a pacemaker or defibrillator, as
well as devices that are used outside the body,
such as a CPAP machine. Not following these
instructions may momentarily interfere with the
operation of other medical devices.
WARNING: Cables
Keep children from playing with the tablet
power cord and wand cable, which can pose
a risk of strangulation. To prevent damage to
cables and the risk of electric shock, prevent
pets, pests and children from chewing on
cables.
Cautions
Caution: Medical Procedures and Dental Work
Before all medical procedures tell the person
administering the procedure that you have the
RNS® System implanted. All medical procedures
and dental work should be performed with
caution. Contact your doctor as soon as
possible if you have questions or suspect your
RNS® System is not working properly after a
medical procedure.
Diagnostic x-rays and diagnostic ultrasounds
may be performed without aecting the RNS®
System.
Caution: Applying Pressure on the
Neurostimulator and Leads
DO NOT press on or play with the implanted
neurostimulator or leads. This may damage
the neurostimulator or leads and result in
stimulation not being delivered until they are
surgically repaired or replaced.
Caution: Magnet
DO NOT drop the magnet onto any hard
surface. The magnet can shatter into small,
sharp pieces that can cut the skin.
Caution: Household Magnets and Magnetic
Bracelets
DO NOT put items that contain magnets within
4 inches of the neurostimulator. Magnets
contained in such products as stereo speakers,
AM/FM radios, power tools, cellular, cordless
and conventional phones, as well as magnets
used therapeutically or worn on the body may
interfere with stimulation. Since it is not always
obvious if an item contains a magnet, refer
to the packaging and instructions that came
with the item for more information. You can
also call the manufacturer of the item and ask
them. Most headsets and earphones available in
stores do not interfere with stimulation, but not
all have been tested.
7
SAFETY INFORMATION

Remote Monitor Manual Model 5106
Caution: Battery Depletion
For continued operation, the neurostimulator needs to be surgically replaced when the battery is
depleted. Your doctor will let you know when the neurostimulator needs to be replaced.
Neurostimulator Replacement Indicator
When your neurostimulator battery is low, the remote monitor will show the following message
on the Home screen: "Your neurostimulator battery has reached its replacement window. Please
contact your physician."
NeuroPace recommends you act on this message and contact your physician about replacing your
neurostimulator.
Caution: Neurostimulator Longevity
High and frequent levels of stimulation reduce
neurostimulator battery longevity.
Caution: Removal and EMI Considerations
Before all medical procedures tell the person
administering the procedure that you have
the RNS® System implanted if any system
components (neurostimulator, leads, lead
fragments or cranial prosthesis) remain
implanted after you stop using the RNS® System.
You could still experience side eects from EMI
if any system parts remain implanted. These
eects may result in stimulation of the brain
tissue and tissue damage resulting in serious
injury or death.
Refer to Electromagnetic Emissions and
Immunity on page 48 for more information.
Caution: Lead Replacement and Leads Left in
Place
The long-term safety associated with leads left
in place without use, replacement of leads, and
lead removal is unknown.
Caution: Other Active Implanted Medical
Devices
RNS® System interactions with other active
implantable medical devices (such as
pacemakers, defibrillators, implanted spinal
cord and peripheral nerve stimulators, cochlear
implants, and vagus nerve stimulators) are not
known. Contact your doctor to discuss your
situation or to answer questions.
Caution: Scuba Diving or Hyperbaric Chambers
DO NOT dive below 10 meters (33 feet) of
water or enter hyperbaric chambers above 2.0
atmospheres absolute (ATA). Such pressures
could damage the RNS® System.
Caution: Patient Population for Which Safety
and Ecacy Have Not Been Established
The safety and eectiveness of the RNS® System
has not been established for:
• People with generalized epilepsy
• People with a seizure focus that cannot be
adequately localized
• Pregnant women
• Nursing mothers
• People under the age of 18
• People with simple partial sensory seizures
only
• People with less than three seizures a month
on average
• People who have more than two epileptic
foci
• People who have not failed two antiepileptic
drugs
Caution: Safety and Eectiveness Beyond 24
Months
The safety and eectiveness of the RNS® System
beyond 24 months is unknown.
8
SAFETY INFORMATION

Remote Monitor Manual Model 5106
Caution: Remote Monitor Tablet
DO NOT use the tablet for any other purpose
except as instructed. The tablet is designed
to operate only as part of the remote monitor.
DO NOT make any changes or adjustments to
the tablet hardware or software. This includes
attaching external devices like a mouse. Any
changes you make that are not part of the
instructions may damage the remote monitor
and may not allow you to send data to the
PDMS database.
Caution: Data Collection
You should collect data from the
neurostimulator as directed by your doctor.
If connected to a Wi-Fi network, the remote
monitor sends data automatically to the
PDMS database. NeuroPace recommends you
collect data from the neurostimulator at least
once a day and maintain a Wi-Fi connection
to allow data to be sent the PDMS database
automatically. By receiving your data on a
regular basis, your doctor will be able to
identify problems and make adjustments. Your
doctor will also be able to determine when
battery power is getting low. If you do not
collect data as directed, your doctor may not be
able to review your data and make adjustments
on a timely basis.
If you are having seizures more frequently or
with greater severity, talk with your doctor
as soon as possible. Your doctor may ask
you to collect data on a more frequent
basis until adjustments can be made to the
neurostimulator settings.
Talk to your doctor about what you should
do if you are unable to collect data from your
neurostimulator as directed.
Caution: Operating Temperatures
DO NOT use the wand or tablet in temperatures
above or below the recommended operating
range (32°F - 95°F). The wand or tablet may
not operate properly at temperatures below
32°F or above 95°F. These devices may also
become warm during normal operation. DO
NOT use them when the room temperature is
above 95°F to avoid discomfort.
Caution: Remote Monitor Setup
DO NOT set up the remote monitor where
people can trip over the cords. The cords
may be tripping hazards, especially for small
children and pets. Tripping over the cords may
damage the remote monitor parts, and may
result in bodily injury. DO NOT rest anything
on the power cord. DO NOT plug the wand
into equipment other than the remote monitor
because it could damage the wand. DO NOT
use a USB cable extension from the remote
monitor to the wand.
DO NOT move the remote monitor to another
location without first disconnecting the
parts and storing them in the carrying case.
Disconnect the wand and all cords from the
tablet. You may damage the parts if you do not
disconnect them before moving them.
Caution: Heating
The remote monitor’s AC adapter may become
hot during normal operation. Use care when
handling during or immediately after operation.
9
SAFETY INFORMATION

Remote Monitor Manual Model 5106
The following diagram illustrates the NeuroPace® Remote Monitor network architecture in the
home environment.
The NeuroPace® Remote Monitor uses a short-
range wireless link between the wand and
the neurostimulator to collect the data stored
on the neurostimulator. The remote monitor
securely uploads this information to the PDMS
using a Wi-Fi connection. Wi-Fi is also used
to download software updates to the remote
monitor. These processes are designed to
reduce cybersecurity risks and protect data
from being read by anyone except those
authorized by the patient.
• Data communicated between the remote
monitor and the PDMS via the Internet are
encrypted using HTTPS (TLS). The remote
monitor will upload patient data only to the
PDMS.
• The remote monitor only runs the
NeuroPace application. The remote monitor
does not have a web browser for other
Internet communications.
• The remote monitor uses Windows firewall
software, which provides strong anti-virus
and anti-malware protection, prevents
unwanted connections and logs security
events and issues.
• The remote monitor deletes a patient’s
Protected Health Information (PHI) after
upload of the information to the PDMS is
complete.
• The remote monitor hard drive is encrypted
with AES-256 security so data cannot be
accessed if the device is lost or stolen.
• NeuroPace maintains regular security
patches for the Windows operating system.
WIRELESS INFRASTRUCTURE
REQUIREMENTS
The NeuroPace® Remote Monitor requires a
Wi-Fi connection to upload data to the PDMS.
This may be through a home wireless router or
another available Wi-Fi network that does not
require a web browser to create an account
for the connection. The network must allow
Internet communications on Port 443 for the
encryption software used between the remote
monitor and the PDMS.
Cybersecurity, Device Security,
and Patient Information
10
CYBERSECURITY

Remote Monitor Manual Model 5106
BACKUP AND RESTORE FUNCTION
Data downloaded from your neurostimulator
is deleted from the remote monitor after it is
uploaded to the PDMS. The remote monitor
does not store therapy settings or other patient-
specific information. Therefore, the remote
monitor does not need to be backed up or
restored and any remote monitor will work with
your neurostimulator.
SECURE USB CONNECTION
The USB port on the remote monitor is
designed to work only with the Wand. Do not
connect anything other than the Wand to the
USB port.
SOFTWARE SECURITY UPDATES
NeuroPace issues software updates periodically
to help secure the RNS System, including the
remote monitor. Software security updates
should be installed as soon as possible. See the
NeuroPace Update Center on page 35 for
more detail.
SECURITY EVENTS
The Windows security software logs security
events and issues but security notifications are
not displayed during normal operation.
If your remote monitor becomes unresponsive
or does not function as expected or if your
remote monitor is lost or stolen, please contact
NeuroPace Customer Support.
11
CYBERSECURITY

INDICATIONS FOR USE
Refer to your RNS® System Patient
Manual for the indications for use. For a
complete discussion of indications for use,
contraindications, warnings, cautions, and
potential side eects, talk to your doctor.
PURPOSE OF YOUR REMOTE MONITOR
The NeuroPace® Remote Monitor (5106) is
designed for use with the RNS® Neurostimulator
(models RNS-300M and RNS-320).
A remote monitor lets you collect data from
the neurostimulator and automatically sends
the data to your doctor. The remote monitor
consists of a special software program installed
on a tablet computer, a wand, and accessories.
After connecting the hand-held wand to the
tablet, data in the neurostimulator are collected
by placing the wand over the neurostimulator.
The wand uses Radio Frequency (RF)
communication to collect the data. Data are
stored in the tablet and then sent to a secure
database. The database is called the PDMS
(Patient Data Management System) and your
doctor can access your data. Your doctor will
review the data and use the results to adjust the
neurostimulator settings during future oce
visits.
IMPORTANT INFORMATION
Please see your RNS® System patient manual
for additional information regarding the RNS®
System and treatment.
You must be willing to collect data from the
neurostimulator as directed by your doctor.
If connected to a Wi- Fi network, the remote
monitor sends data automatically to the PDMS
database. NeuroPace recommends you collect
data from the neurostimulator at least once
a day and maintain a Wi-Fi connection to
allow data to be sent to the PDMS database
automatically.
About the RNS® System
The RNS® System Patient Manual
has a cover that looks like this.
13
ABOUT

Remote Monitor Manual Model 5106
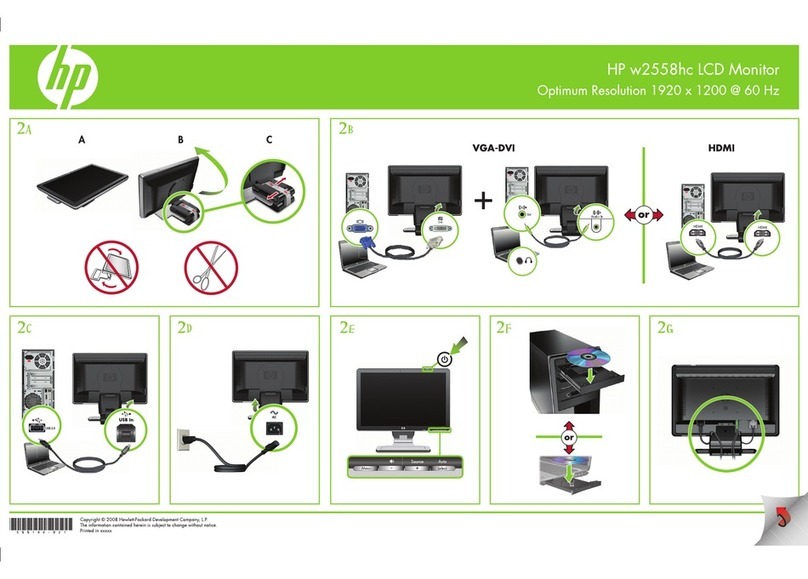
Make sure you have all the parts described below.
Contact NeuroPace Customer Support at 866-726-3876 if any parts are missing.
Parts of the NeuroPace® Remote Monitor
1 CARRYING CASE
The carrying case is for storing your remote monitor parts when not in use.
2 THE REMOTE MONITOR SOFTWARE ON TABLET COMPUTER
The remote monitor software program is already installed on the tablet computer. It stores
data collected by the wand, and then sends it to the secure NeuroPace database (PDMS).
The tablet computer you receive may not be the same model shown here. The location of
the connections may also dier from the model shown here.
3 WAND
The Wand plugs into the tablet computer and is applied to the patient (an applied
part). You hold it over the neurostimulator to collect data from the neurostimulator and
store it in the tablet computer. The wand collects data through Radio Frequency (RF)
communication.
4 POWER CORD
The power cord powers the tablet computer and charges its battery. The tablet comes with
the battery installed, but not fully charged. When the battery is charged, disconnect the
power cord before using the wand. The power cord may come as 1 or 2 pieces, depending
on the tablet computer model you receive.
1
2
3
4
The tablet computer you
receive may not be the same
model shown here. The
location of the connections
may also dier from the
model shown here.
15
PARTS

FIND A GOOD PLACE TO SETUP
The remote monitor is intended to be used by the patient at home. Follow the steps below
to set up the remote monitor for use. Contact NeuroPace Customer Support if you need
assistance to set it up or to report any unexpected events.
Review the Safety Information on page 4 before continuing.
Find a suitable place to set up the remote monitor. This should be an area:
• Away from water, moisture or dampness that
can damage the wand and tablet.
• Away from extreme temperatures (below
32°F or above 95°F) that can interfere with
wand and tablet operation.
• Away from small children and pets who can
damage the wand and tablet.
• Away from large electrical appliances that
might be a source of electromagnetic
interference (EMI) and interfere with wand
and tablet operation.
• Near an electrical outlet that will accept the
type of power cord plug that came with your
tablet.
• In range of your Wi-Fi network.
You will not be able to confirm that there are no
large sources of EMI nearby until you observe
the wand signal while collecting data.
Refer to Get Data from Your Neurostimulator
on page 27.
Setup the Remote Monitor
1 Locate the power connection on your
tablet. Plug one end of the power cord
into the tablet and the other end into
an electrical outlet. You may need an
outlet that accepts a 3-pronged plug. If
your power cord comes as 2 separate
pieces, first attach the 2 pieces before
connecting the tablet to the outlet.
2 Locate the USB port on your tablet. Plug
the wand cord into the USB port. The
image below shows the remote monitor
tablet with all connections made while
charging.
1 2
The tablet computer you
receive may not be the same
model shown here. The
location of the connections
may also dier from the
model shown here.
CHARGE THE REMOTE MONITOR
17
SETUP

Note: Before using the wand, make sure the tablet is
suciently charged and unplug it from the electrical outlet.
The remote monitor is ready for use when the wand is
connected, the battery is charged and the power cord is
removed. See image below.
Unplug
before
use.
Ready
for
use!
The tablet computer you
receive may not be the same
model shown here. The
location of the connections
may also dier from the
model shown here.
18
SETUP
Other manuals for 5106
1
Table of contents
Other Neuropace Monitor manuals