Spectramed Aspire2 User manual
120*175mm
Date: 2016/12/23
sEMG Biofeedback FES
Neuro Muscular Electrical Stimulator
01AS80 User manual
Thanks for buying our product
Please carefully read the user manual before use and keep the manual in a safe place.

Table of Contents
Chapter 1 Introduction
Product Summary
Main innovations
Design Principles
Chapter 2 General Warnings and Precautions
Indications for use
Contraindications
Warnings
Precautions
Adverse Reactions
Skin Care
Chapter 3 The Aspire 2
Description
Features
Settings
Chapter 4 Operation
Power on
Mode Selection
Other Functions
Chapter 5 Care and Maintenance
Stimulator
Battery
Lead Wires
Guardian Self-Adhesive Electrodes
Chapter 6 Specifications and Parameters
Chapter 7 Manufacturer's Declaration
Warranty
Customer Service
01
01
01
02
04
04
04
04
05
06
06
07
07
07
08
09
09
09
13
15
15
15
16
16
17
18
22
22

01 22
Chapter 1 Introduction
1.1 Product Summary
The Aspire2 is an advanced sEMG biofeedback and electrical stimulation com-
bination device with gaming technology designed for the rehabilitation needs of
people with disorders that impact nerve and muscle function.
The stimulator will be utilized for the rehabilitation of muscle function. The stimulator
detects and monitors the weak EMG signal of patient and delivers an electrical
pulse according to EMG signal strength to stimulate the patient to achieve a muscle
contraction. With multiple training modes and interactive gaming applications,
patients can actively participate in the rehabilitation process and receive the treat-
ment with greater enjoyment and personalization. The stimulator is also equipped
with an evaluation function to establish baseline data and threshold as well as
track rehabilitation progress to help medical professionals customize evidence
based, objective and effective rehabilitation treatment programs for each patient.
1.2 Main Innovations
1. Technology of self-EMG signal acquisition and processing;
2. New generation of real-time biofeedback functional electrical stimulation
technology based on EMG signal and muscle strength;
3. iPad interactive games training and rehabilitation therapy applications (APP);
4. Multiple rehabilitation training modes in the APP.
Warranty
Spectramed, Inc. provides a warranty to the original purchaser that this product
will be free from defects in the material, components and workmanship for a period
of 1 years from the date of purchase [invoice date]. If Spectramed, Inc. are satisfied
that the product/s is defective the purchaser may return this unit/s to Spectramed,
Inc. or the appointed distributor for repair or replacement with a new unit. All returns
must first be authorized by Spectramed, Inc. in advance. The liability of Spectramed,
Inc. under this limited product warranty does not extend to any misuse or abuse
such as dropping or immersing the unit in water or other liquid substance or tamper-
ing with the unit or normal wear and tear. Any evidence of tampering will nullify this
warranty.

21 02
1.3 Design Principles
The stimulator detects the motor unit action potential (MUAP) elicited during a
muscle contraction through the surface electrodes attached to the patient's body
part, extracts its active EMG signal and muscle strength after filtering, and then
simultaneously delivers low frequency electrical stimulation according to the
muscle strength exerted. The stimulation evokes an action potential causing a
muscle contraction that is stronger than the patient could achieve without the
stimulation. The patient is actively engaged in the training process through bio-
feedback technology. Please refer to chart 1 to 4.
Chart 1: The stimulator detects EMG signal through electrodes, and extracts muscle
strength by amplification and filtration, and then applies functional electrical stimulation
to the arm.
Chart 2: Leg EMG feedback functional electrical stimulation.
•
•
•
•
It is customer responsibility to assure that this equipment and
vicinity equipment complies the value of radio frequency inter-
ferences shown in General Regulation for safety according to
IEC 60601-1-2 Tables as described in this section.
The manufacturer is not responsible for any interference caused
by using other than recommended interconnect cables or by
unauthorized changes or modifcations to this equipment.
Important Notice
The Aspire 2 meets the requirement of electromagnetic compatibility in IEC60601-1-2.
The user needs to install and use according to electromagnetism compatibility
information which is attached with it.
Portable and mobile RF communication devices may influence the Aspire 2
performance, so the Aspire 2 should be kept away from them during using.
Guidance and manufacturer's declaration stated in the appendix.
Warning:
•The Aspire 2 should not be used adjacent to or stacked with other equipment and
that if adjacent or stacked use is necessary, the Aspire 2 should be observed to
verify normal operation in the configuration in which it will be used.

03 20
Chart 3: Paraplegic patients' EMG feedback functional electrical stimulation (Paraly-
sis of the legs) .
Chart4: EMG feedback functional electrical stimulation for 2 kinds of forearm move-
ments.
RECOMMENDED SEPARATION DISTANCES BETWEEN PORTABLE AND
MOBILE RF COMMUNICATIONS EQUIPMENT
AND THE PORTABLE ELECTRICAL STIMULATOR UNIT
This Aspire 2 is intended for use in an electromagnetic environment in which radiated RF disturbances
are controlled. The customer or the user of this Aspire 2 can help prevent electromagnetic interference
by maintaining a minimum distance between portable and mobile RF communications equipment
(transmitters) and this Aspire 2 as recommended below, according to the maximum output power of
the communications equipment.
Rated maximum output Separation distance according to frequency of transmitter
power of transmitter m
W 150 KHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.7 GHz
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance ’d’
in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where ’P’ is
the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 - At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 - These guidelines may not apply in all situations. Electromagnetic propagation is afected by
absorption and refection from structures, objects and people.
d =
1.2 d = d =
Returning Equipment for Repair
Be sure to call for an authorization number (RA) before returning any equipment.
Send the unit(s) postage prepaid and insured, with proof of purchase, to the
address below. If you are shipping from outside the United States, mark the
package ‘Goods to be repaired-Made in China’ to avoid unnecessary customs
charges.
Provide a detailed description of the problem you are experiencing, along with your email,
phone number and fax number if applicable.
Technical Support and Repair
1.2 2.3
Any queries should be addressed to Spectramed at:275 W Johnstown Rd
Gahanna, OH 43230

19 04
Chapter 2 General Warnings and Precautions
Please carefully read the user manual before use. If you have any
questions please contact your physician, rehabilitation therapist
or your training manual before proceeding.
Indications for Use
* Prevention or retardation of disuse atrophy
* Relaxation of muscle spasms
* Increasing local blood circulation
* Immediate post-surgical stimulation of calf muscles to prevent venous throm-
bosis
* Maintaining or regaining range of motion
* Biofeedback
* Detecting and extracting an active EMG signal
Contraindications
1) Do not use this device on patients who have a cardiac demand pacemaker,
implanted defibrillator, or other implanted metallic or electronic device because
this may cause electric shock, burns, electrical interference, or death.
2) Electronic muscle stimulation stimulators, including this unit, should not be
used on patients with any form of cancer.
* Muscle re-education
List of Symbols
Type BF Applied Part(s)
Caution
Consult Instructions for Use
Portable and mobile RF communications equip-
ment should be used no closer to any part of this
Aspire 2 including cables, than the recommended
separation distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance
d = 1.2
d = 1.2 80 MHz to 800 MHz
d = 2.3 800 MHz to 2.7 GHz
where ‘P’ is the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and ‘d’ is the
recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey a),
should be less than the compliance level in each
frequency range b). Interference may occur in the
vicinity of equipment marked with the following
symbol:
MANUFACTURER’SDECLARATION–ELECTROMAGNETICIMMUNITY
This Aspire 2 is intended for use in the electromagnetic environment specified below. The customer or the
user of this Aspire 2 should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Conducted RF 3Vrms
IEC 61000-4-6 150 kHz to 80 MHz
Radiated RF 3 V/m
IEC 61000-4-3 80 MHz to 2.5 GHz
NOTE 1 - At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 - These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and refection from structures, objects and people.
a) Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones
and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted
theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an
electromagnetic site survey should be considered. If the measured field strength in the location in which
this Aspire 2 is used exceeds the applicable RF compliance level above, this Aspire 2 should be observed
to verify normal operation. If abnormal performance is observed, additional measures may be necessary,
such as re-orienting or relocating this Aspire 2.
b) Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 30 V/m.
Chapter 7 Manufacturer's Declaration
6Vrms
3 V/m

05 18
9) Apply stimulation only to normal, intact, clean, healthy skin.
10) Do not apply stimulation across the patient's chest because the introduction
of electrical current into the chest may cause rhythm disturbances to the
patient's heart, which could be lethal.
11) This unit must be used with the guidance of a physician, speech therapist,
speech language pathologist, occupational therapist or physical therapist.
12) Do not insert lead wires into a mains power supply.
13) Do not immerse unit into water or any other substance.
14) Do not use this device in the presence of a flammable anesthetic gas mixture
and air or with Oxygen or Nitrous Oxide.
15) Plug the lead wire out of the device and do not use while charging.
16) Patient Electrodes are for single patient use only.
17) Keep out of reach of children.
18) Do not apply stimulation over, or in proximity to, cancerous lesions.
19) Do not apply stimulation while the patient is driving, operating machinery,
or during any activity in which electrical stimulation can put the patient at
risk of injury.
Precautions
Precautions should be observed in the presence of the following
1) When there is a tendency to hemorrhage following acute trauma or fracture.
2) Following recent surgical procedures when muscle contractions may disrupt
the healing process.
5) Do not apply stimulation in the presence of electronic monitoring equipment
(e.g., cardiac monitors, ECG alarms), which may not operate properly when
the electrical stimulation device is in use.
6) Do not apply stimulation when the patient is in the bath or shower.
7) Do not apply stimulation while the patient is sleeping.
8) Consult with the patient's physician before using this device because the device
may cause lethal rhythm disturbances to the heart in susceptible individuals.
4) Stimulation should not be applied over swollen, infected, or inflamed areas or
skin eruptions, e.g., phlebitis, thrombophlebitis, varicose veins, etc.
Warnings
1) The long-term effects of electrical stimulation are unknown.
2) Stimulation should not be applied transthoracically in that the introduction of
electrical current into the heart may cause cardiac arrhythmias.
3) Stimulation should not be applied over the carotid sinus nerves, particularly in
patients with a known sensitivity to the carotid sinus reflex.
3) Use caution if stimulation is applied over the menstruating or pregnant uterus.
4) Where sensory nerve damage is present by a loss of normal skin sensation.
This Aspire 2 uses RF energy only for its internal
function. Therefore, its RF emissions are very low
and are not likely to cause any interference in
nearby electronic equipment.
The Aspire 2 is suitable for use in all establishments,
including domestic establishments and those directly
connected to the public low-voltage power supply
network that supplies buildings and homes used for
domestic purposes.
MANUFACTURER’S DECLARATION - ELECTROMAGNETIC EMISSIONS
This Aspire 2 is intended for use in the electromagnetic environment specified below. The customer or the
user of this Aspire 2 should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11 Group 1
RF emissions
CISPR 11 Class B
Harmonic emissions
IEC 61000-3-2 Class A
Voltage fuctuations/
Flicker emissions
IEC 61000-3-3
Complies
MANUFACTURER’S DECLARATION - ELECTROMAGNETIC IMMUNITY
This Aspire 2 is intended for use in the electromagnetic environment specified below. The customer
or the user of this Aspire 2 should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient burst
IEC 61000-4-4-
Surge
IEC 61000-4-5-
Voltage dips,
short inter-
ruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
Power frequency
(50/6- Hz)
magnetic field
IEC 61000-4-8
±6 kVcontact
±8 kVair
±2 kVfor
power supply
lines
±2kVline(s)
and neutral
<5% U
T
(>95% dip in U
T)
for 0.5 cycle
40& U
T
(60% dip in U
T)
for 5 cycles
70% U
T
(30% dip in U
T)
for 25 cycles
<5% U
T
(>95% dip in U
T)
for 5s
3 A/m
±8kVcontact
±15kVair
Mains power quality should be that of a typical
commercial or hospital environment.
Mains power quality should be that of a typical
commercial or hospital environment.
Power frequency magnetic fields should be at
levels characteristic of a typical location in a
typical commercial or hospital environment.
NOTE: U
T is the a.c. mains voltage prior to application of the test level.
Floors should be wood, concrete or ceramic tile.
If floors are covered with synthetic material, the
relative humidity should be at least 30 %.
±2 kV for power
supply lines
± 1 kV line(s) to
line(s)
<5 % UT
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 s
3 0A/m
Mains power quality should be that of a typical
commercial or hospital environment. If the user
of the (Name or model) requires continued opera-
tion during power mains interruptions, it is re-
commended that the (Name or model) be
powered from an uninterruptible power supply
or a battery.

17 06
10) Discontinue and do not increase the intensity level if you feel discomfort during use.
11) Stimulation should not be applied transcerebrally.
12) Stimulation should be stopped if discomfort is felt or reported.
13) The safety of electrical stimulation during pregnancy has not been established.
14) Patients with suspected or diagnosed heart disease should follow precautions
recommended by their physicians.
15) Patients with suspected or diagnosed epilepsy should follow precautions re-
commended by their physicians.
Adverse Reactions
* Skin irritation and burns beneath the stimulation electrodes have been reported
with the use of powered muscle stimulators.
Skin Care
* Skin should be clean and free of any perfumes, alcohol, lotion or debris.
* Skin prep pads may be used during NMES mode but may interfere with the
sEMG signal for EMG testing or ETS mode.
* Presence of facial hair may interfere with electrode function however skin may
be very sensitive after shaving so it is recommended to wait several hours
after shaving before applying electrodes to avoid irritation.
* Gently remove electrodes from the skin.
Chapter 6 Specifications and Parameters
Power: rechargeable lithium battery 7.4V
Safety class: internal power BF type
Protection type: Class II device
Shutdown current: < 0.1mA
Operating current: 0 ~60mA
Measuring range: 10uV ~999uV
Highest resolution: < 2uV
Input noise: <10uV
Transmission bands: 120Hz ~ 1000Hz (-3dB)
Differential mode input impedance: >5MΩ
Common mode rejection ratio: >100dB
NMES
Frequency, 5-100Hz, biphasic balanced wave ±10%
Pulse width: 50 ~ 450uS (±10%)
Output intensity: load 1000Ω, min 1mA, max 60mA
ETS
Frequency: 18 Hz, biphasic balanced wave ±10%
Pulse width : 150 uS (±10%)
Output intensity: load 1000Ω, min 1mA, max 60mA
Working Environment
Temperature: 5℃~ 40 ℃
Relative humidity : ≤80%HR
Atmos.: 86Kpa ~ 106Kpa
Storage Environment
Temperature : -20℃~ 55 ℃
Relative humidity : ≤93%HR
Atmos.: 70Kpa ~ 106Kpa
Device size: 112mm*56mm*18mm
Device weight: 93g
6) Electrode placement and stimulation settings should be based on the guid-
ance of a physician, speech therapist, speech-language pathologist, occu-
pational therapist or physical therapist.
5) Some patients may experience skin irritation or hypersensitivity due to the
electrical stimulation or electrical conductive medium. The irritation can
usually be reduced by use of an alternate conductive medium, or alternate
electrode placement.
7) Powered muscle stimulators should be used only with the leads and electrodes
recommended for use by the manufacturer.
8) Since the effects of stimulation of the brain are unknown, stimulation should
not be applied across the head, and electrodes should not be placed on
opposite sides of the head.
9) Use caution applying stimulation over the patient's neck because this could
cause severe muscle spasms resulting in closure of the airway, difficulty in
breathing, or adverse effects on heart rhythm or blood pressure.
* Patients should stop using the device and consult their physicians if they
experience adverse reactions with the device.
* Patients may experience headache and other painful sensations during or
following the application of electrical stimulation near the eyes and to the face.
07 16
Chapter 3 The Aspire 2
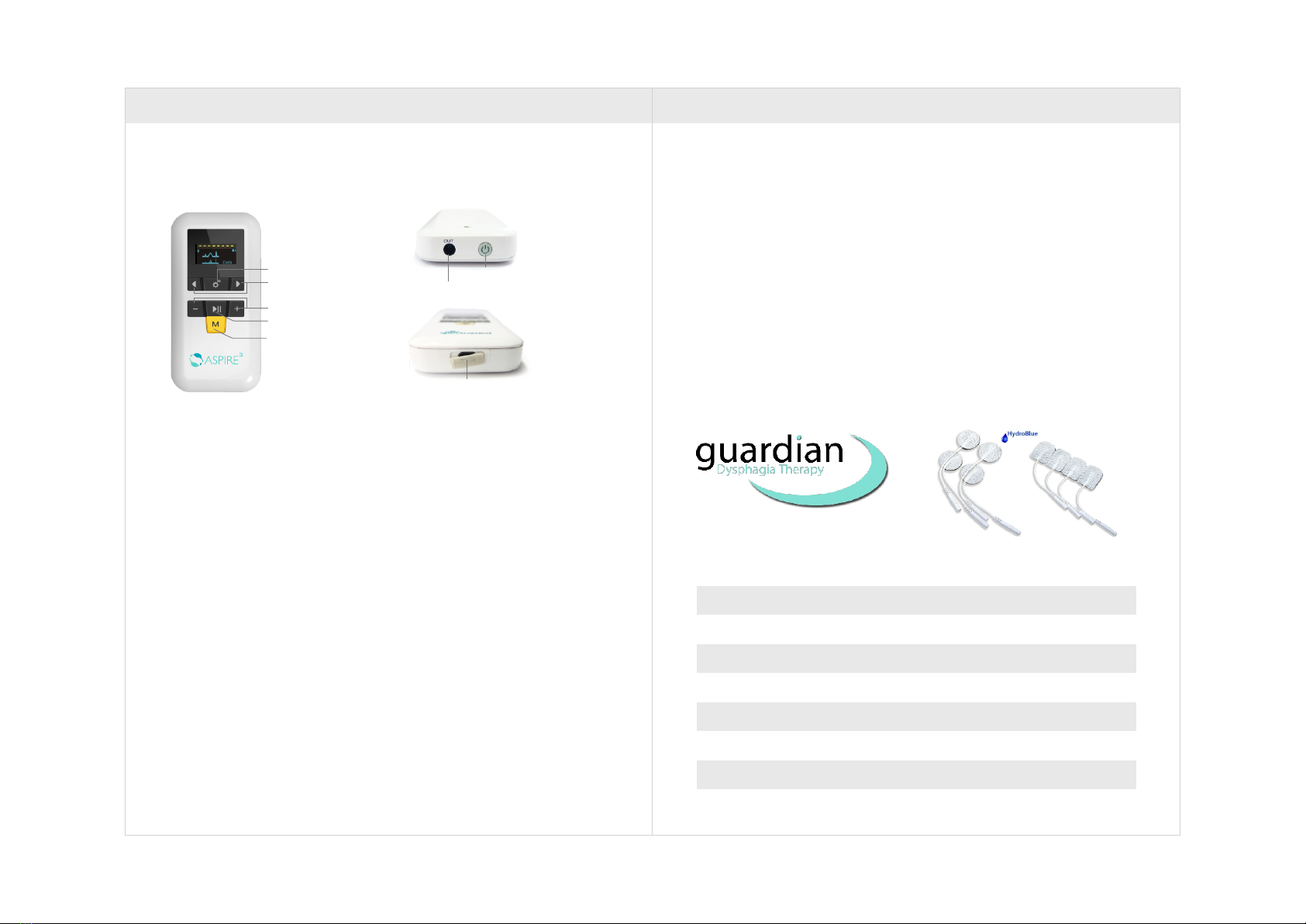
3.1 Description
6. Power Button
7. Stimulation output/EMG input port
8. Micro USB port
3.2 Features
1. OLED screen
2. ETS (EMG Triggered Stimulation) function
3. NMES (Neuromuscular electrical stimulation)
4. Multimedia interactive training (Endurance training, coordination training,
strength training)
5. Functional electrical stimulation prescription management
6. Electrodes Isolation technology: ETS and NMES mode use 1 electrode wire
7. Bluetooth 4.0 (communication with iPad)
8. Electrodes loss detection function
9. Low battery warning function
10. Symmetric Biphasic Balanced wave
11. Rechargeable lithium battery + micro USB port
12. Data storage function
13. Usage time lock function
14. ETS and NMES mode are available without iPad
Lead Wires
* The lead wires should be handled carefully and never stretched, as this can
cause the stimulation to function below normal standards or not at all.
* Examine lead wires before each treatment for loose connections or damage.
* Avoid stretching and twisting the lead wires.
* Store the lead wires carefully after each use.
Guardian Self-Adhesive Electrodes
* Check the short connectors to make sure they have not become separated
from the electrodes.
* Replace electrodes onto plastic film after use. If they drop onto the floor, debris
will adhere to conductive gel rendering the electrodes ineffective.
* Optimal electrode function cannot be guaranteed after 4 uses.
* Keep electrodes away from water.
* Try not to touch adhesive portion of electrode as this can interfere with the
integrity of the conductivity and equal distribution of current.
* If an allergic reaction is obsessed or reported stop using the device and seek
medical attention.
1. Setting button
2. Selection button
3. Play/Pause button
4. Intensity Button
5. Mode Button
To reorder Guardian electrodes
Please visit us at: www.spectramedonline.com
Part #
05A156
05A158
05A159
05A160
05A161
05A162
05A163
Shape/
Dimension
1.0" Round
.875" Round
1.0"x1.25"
Rect.
1.0" Round
1.0"x1.25"
Rect.
1.25" Round
.875" Round
Connection
Wire
Wire
Wire
Wire
Wire
Wire
Wire
Top
Laminate
Non Woven
Cloth
Non Woven
Cloth
Non Woven
Cloth
Non Woven
Cloth
Non Woven
Cloth
Non Woven
Cloth
Non Woven
Cloth
Hydrogel
HydroBlue
Gel
HydroBlue
Gel
HydroBlue
Gel
Standard
Hydrogel
Standard
Hydrogel
HydroBlue
Gel
Standard
Hydrogel
15 08
3.3 Settings
It is used to set the prescription. In NMES mode, press , the selected pre-
scription will blink, press or to select the desired prescription, press
again to confirm the selection.
3.3.1 Setting/Enter Button
3.3.2 Selection Buttons
In NMES mode, press or to select prescription, there are 5 default
prescriptions for selection. For the 5 default prescriptions, the first 4 are fixed,
and 5th can either be downloaded from the APP or manually set through the
stimulator.
3.3.3 Play/Pause button
In ETS or NMES mode, Press to start training, press again to pause.
Note: is only used in “ETS” or “NMES” mode.
3.3.4 Intensity Adjustment Buttons
In ETS or NMES mode, the initial current is 0 mA, press to increase the
intensity, press to decrease the intensity.
At 0-5 mA, the current will increase in 1mA increments;
At 5-21 mA, the current will increase in 0.5mA increments;
At 21 mA and more, the current will increase in 0.1mA increments.
3.3.5 Mode Button
When the stimulator is turned on, it automatically defaults to ETS mode,
press , to switch among ETS, NMES and GAME.
3.3.6 Power Button
Press and hold on for 2 seconds to turn on the stimulator, press again to
turn off the stimulator. When the stimulator is in charging, press once to show
battery level.
3.3.7 Stimulation output/EMG input port
It is for electrical stimulation output and EMG input collection.
Chapter 5 Care and Maintenance
Stimulator
* Wipe the surface once a week with a slightly water dampened cloth or antiseptic wipe.
* Do not use cleaning sprays or alcohol based cleaning solutions.
* Do not store in direct sunlight, in high temperatures, moist areas, dusty areas or
near corrosive gas.
* Do not immerse in water.
* Do not throw, step on or exert pressure on the stimulator.
* When the stimulator reaches the end of its service life (3 years), please dispose
of it in accordance with the local and national regulations.
Battery
The Aspire2 is a rechargeable stimulator with a universal recharging USB cable.
The cable can be used to recharge your stimulator through a computer USB port
or the provided power adapter.
Any attempt to replace the batteries or charge the stimulator differently than
instructed in this manual may result in overheating of the battery and or fire.
To recharge the stimulator:
1) Open the connection port cover located on the bottom of the stimulator,
2) Connect the recharging cable to the stimulator,
3) Plug the other end into the USB port on your computer or place the adapter
onto the end of the cable and plug into a 110 Volt wall outlet,
4) When finished close the connection port cover,
5) The stimulator is now ready to use again.
The stimulator is designed to work for Ten (10) hours with a full charge.
The stimulator will need to charge for about Three (3) hours when completely
drained of power.
Note: Do not use the device while charging.
Battery specification
Type: Polymer Lithium-Ion Battery
Model: E423150
Capacity: 650 mAh
Voltage: 7.4V
Power Adapter
Type: Medical Power Supply
Model: GTM41078-0605-USB
Input: 100-240V, 50-60Hz, 0.3A
Output: 5V 1.2A
The power adapter conforms to the standards of IEC 60601-1.
* NOTE: you must first press the " " in NMES & ETS mode before pressing
the " " to increase intensity.

09 14
Chapter 4 Operation
4.1 Power on
Press the power button and hold for 2 seconds to enter into the interface as below:
4.2 Mode Selection
4.2.1 ETS
EMG Triggered Stimulation (ETS) is a therapeutic fusion between sEMG Bio-
feedback and NMES. The stimulator delivers electrical stimulation according
to the EMG signal of the body part. During ETS, the patient is required to co-
ntract the muscle as much as possible against a target threshold. Once the
target is achieved, the stimulation is triggered to against a target threshold.
Once the target is achieved, the stimulation is triggered to supplement the
naturally achieved muscle contraction beyond the contracting muscle's actual
ability. There are 2 ways to achieve a threshold of stimulation: 1. The user
manually downloads the threshold from the iPad APP or; 2. The stimulator dete-
cts the EMG signal and sets a threshold based on the signal: the screen will
display “Relax” and then “Flex”, after which the threshold will be set.
The default mode is ETS once the stimulator is powered on. The user can down-
load a threshold from the iPad APP or the stimulator will work on the default thres-
hold, the threshold is displayed in the middle of the yellow area. When down-
loading 0 from the iPad APP, AUTO will be displayed in the middle of yellow area.
4.3.4 Time Locking
The treatment time can be downloaded from the APP and the stimulator will
operate within the specified time, once the prescribed time is up, the stimulator
will shut down and cannot work again until downloading another treatment time
from the APP.
4.3.5 Current Limit
A current limit value can be downloaded from the APP to limit the maximum output
current. The available working current of the stimulator is 0 to 60 mA, however, if
the user downloads a current limit value of 25 mA from the APP, then the maximum
output current will stop at 25mA.
When using stimulation modes NMES or ETS. As a safety feature the user must
first press the “ -“ button twice beforepressing the “ +” key to increase intensity.
This is to ensure that an unwanted increase in “mA” intensity does not occur. The
stimulator is designed to automatically lock after 10 seconds. To unlock and increase
intensity the user must first press the “-“button twice and then proceed to pressing
the “+” button to increase intensity to the desired setting.
4.1.1 Safety Feature
4.3.2 Loose electrode warning
When the electrodes are not properly contacted with the skin, the output will
stop and the screen will display a electrodes loose warning. Please check the
electrodes and reattach them, making full contact with the skin, then press
to start again. The followings are the conditions that will cause a electrodes
loose warning:
1. In ETS or GAME mode, if any of the three electrodes are loose
2. In NMES mode, if any of the yellow electrodes are loose
4.3.3 Data Storage
The data which can be stored in the system includes: current limit value, previ-
ously used prescription, previously downloaded threshold, the rest time for treat-
ment; and parameters for each prescription in NMES mode: frequency, pulse
width, ramp up, ramp down, duration, interval, etc.

13 10
Setting Threshold
Patients will demonstrate varied levels of EMG activity and thus, the effects of
doing ETS may be different between patients. Optimal effects can be achieved
if the user downloads an appropriate threshold from the APP.
Download “0” from the iPad, the digital display will read AUTO in the center of
the yellow area.
1) Press
2) Stay relaxed for 6 to 8 seconds.
3) Stay flexed for 8 to 10 seconds. The stimulator will then set the threshold.
4.3 Other Functions
When battery power is lower than 20%, the battery icon on the OLED will flash
for 1 second to promote recharging; when battery is lower than 10%, the icon
will flash for 0.5 second and electrical stimulation will shut off; when battery is
lower than 5%, the stimulator will automatically shut down.
4.3.1 Low battery warning
4.2.3 GAME
GAME is the training mode that combines sEMG biofeedback treatment with
interactive gaming technology. Biofeedback is the use of sEMG signals to
provide a real time visual display in ether a graph or animated format of the
muscular effort exerted by the patient. This external feedback helps the patient
gain greater awareness of the effort exerted and gives them a tangible goal to
work towards thereby improving their performance and affecting a physiological
change. The animations provide an enjoyable, engaging format during the
rehabilitation process. Different games have been designed specifically to
address 3 different types of muscle function; strength, endurance and coordina-
tion. The system transfers the collected EMG signal to the iPad through Blue-
tooth technology to control the games.
mA
23.0
mA
23.0
mA
0.0
ETSETS
500
AUTO
500
AUTO
Relax:
1
500
AUTO
Relax:
OK!
500
AUTO
Flex:
3
500
AUTO
Flex:
OK!
Then press , and the stimulator starts to work. Please see following interface:
ETS Mode Start to work
The initial current is 0 mA for security. User can press or to adjust the
intensity.
ETS
500
ETS
500
mA
0.0
1. Press or to switch to the fifth prescription, press to enter the setting page.
2. Press or to select parameters, below are the adjustable parameters:
RQ: frequency
PD: pulse width
CT: duration of stimulation
RT: rest time between 2 stimulations
RU: ramp up
RD: ramp down
Times: Cycles of stimulation
3. Press or to adjust the parameters.
4. Press to confirm.
Manually set the customized prescription
Note: If user downloads the program from iPad, the fifth program will display as
P1 to P42 If user customizes the program by the stimulator, the fifth program will
display as C.
*

11 12
4.2.2 NMES
Neuromuscular Electrical Stimulation (NMES) is an electrical stimulation training
mode. NMES provides an electrical impulse through surface electrodes to the
peripheral nerves to evoke an action potential. Repeated assisted contractions
of a weak, dysfunctional neuromuscular system results in stronger, more efficient
and coordinated muscle movement. The iPad APP has 4 fixed prescriptions and
1 customized prescription (Custom settings by medical professionals according
to patients' actual conditions) for different body parts based on international
common standards of electrical stimulation and our company's independent study.
sEMG TEST
The sEMG test in the APP detects the patient's EMG signal and provides a sum
of the activity measured in microvolts and a display of the results. This objective
data can be used to establish baseline and progress over time. The threshold is
set in “Setting” by comparing the test results.
After setting, the threshold will be recorded by the system. If user doesn't set
threshold, the system will work with the previously set threshold.
Swallowing sEMG TEST
The swallowing sEMG test detects the EMG signal during a swallow and provi-
des a visual display of the average microvolts. sEMG for swallowing is very
different than sEMG for other muscle movements as the reliability of the signal
will be directly impacted by the type of swallow elicited such as; dry swallow,
5cc water, 10cc water, puree or solid bolus. Proper use of sEMG can be very
useful in providing objective data for establishing baseline and progress over
time. Improper use of sEMG will not yield useful or accurate data.
The stimulator has 5 prescriptions as follows:
4 fixed prescriptions and 1 customized which can either be downloaded from
the APP or manually set through the stimulator.
The following are the 4 fixed prescriptions, each one lasts about 30 minutes:
After selecting a prescription, press to start training.
The initial current is 0 mA for security. User can press or to adjust the
intensity.
Proper use of NMES requires that the stimulus is modified and manipulated
according to the patient's individual needs, the 4 prescriptions provided may
work for the majority of patients but in many cases the stimulus must be
customized for an optimal response. A custom prescription can be created
by connecting the stimulator to iPad, clicking on the 5th prescription and
entering the parameters needed.
Load customized prescription
-A1
NMES
-A2
NMES
-A3
NMES
-A4
NMES
-P4
NMES
-A1
14.5mANT: 520
RT: 800 -A1
14.5mANT: 520
RT: 800
-A1
NMES
Fixed Prescription
Customized Prescription
4) After the threshold is set, the digital display will show the current and the
user can increase to the appropriate current level and start the training
process.
mA
23.0
mA
23.1
*
This manual suits for next models
1
Table of contents
Other Spectramed Fitness Equipment manuals