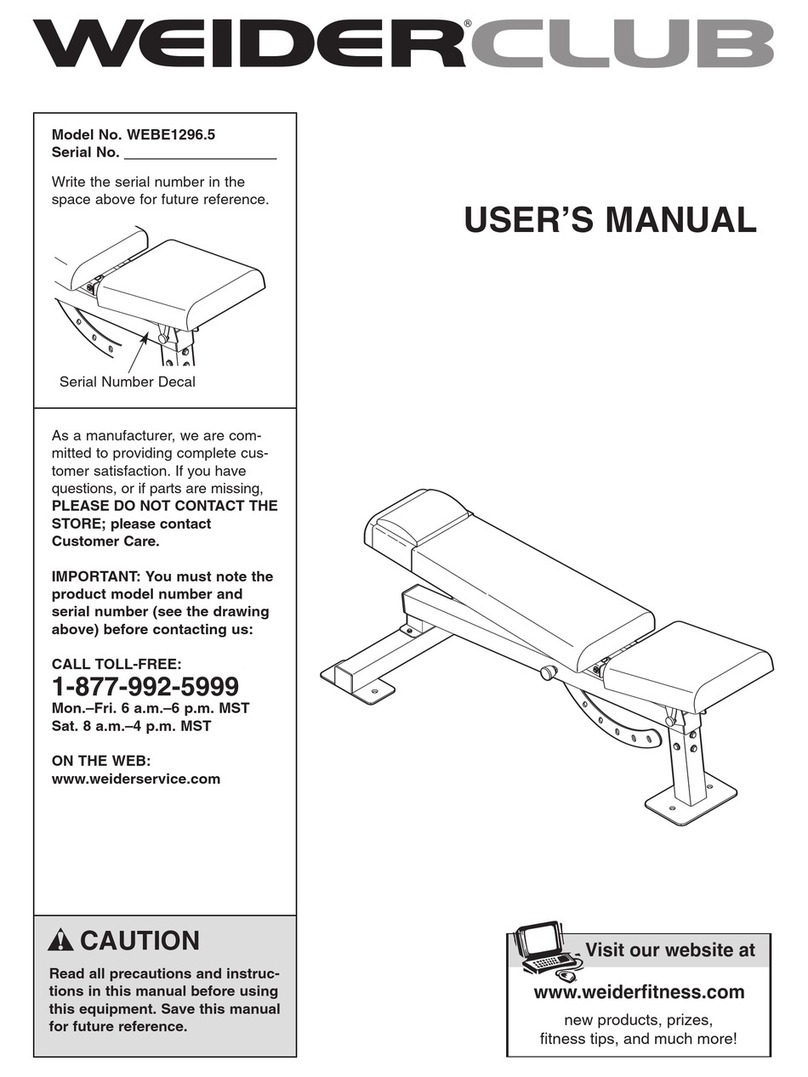
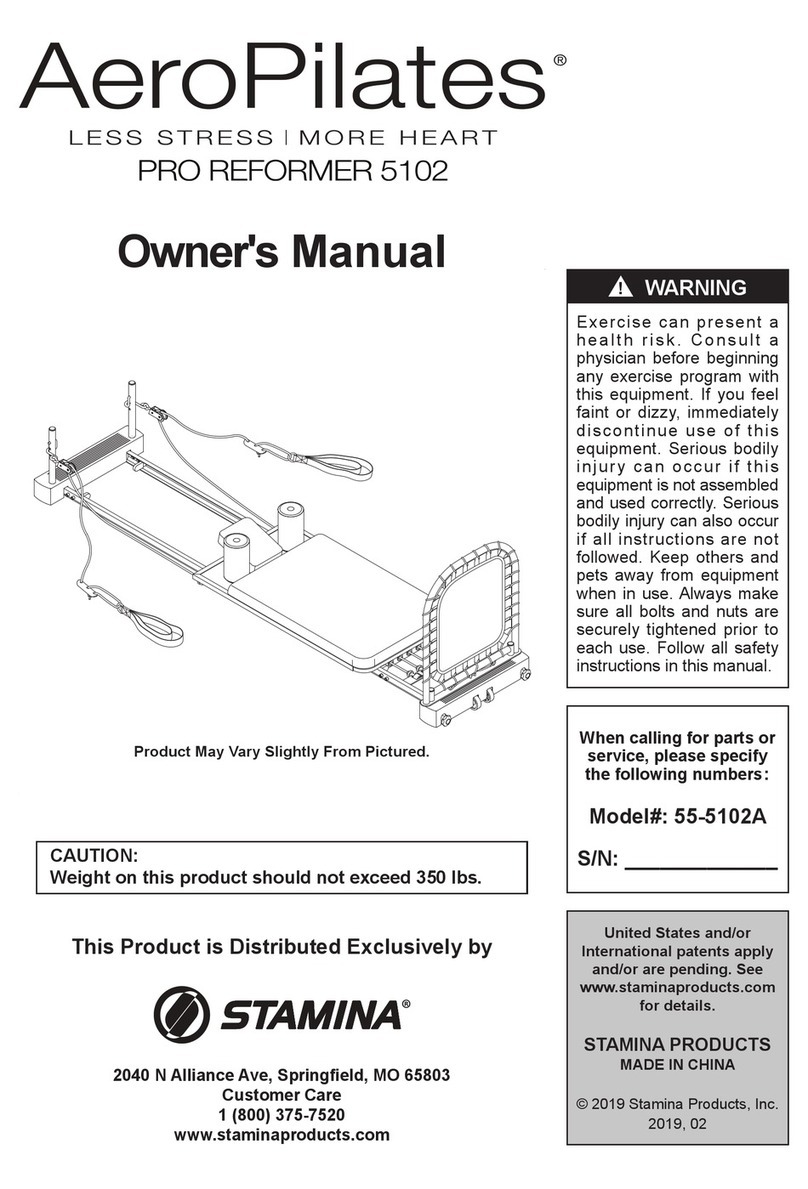
Spectramed Classic SwallowStim Configuration guide

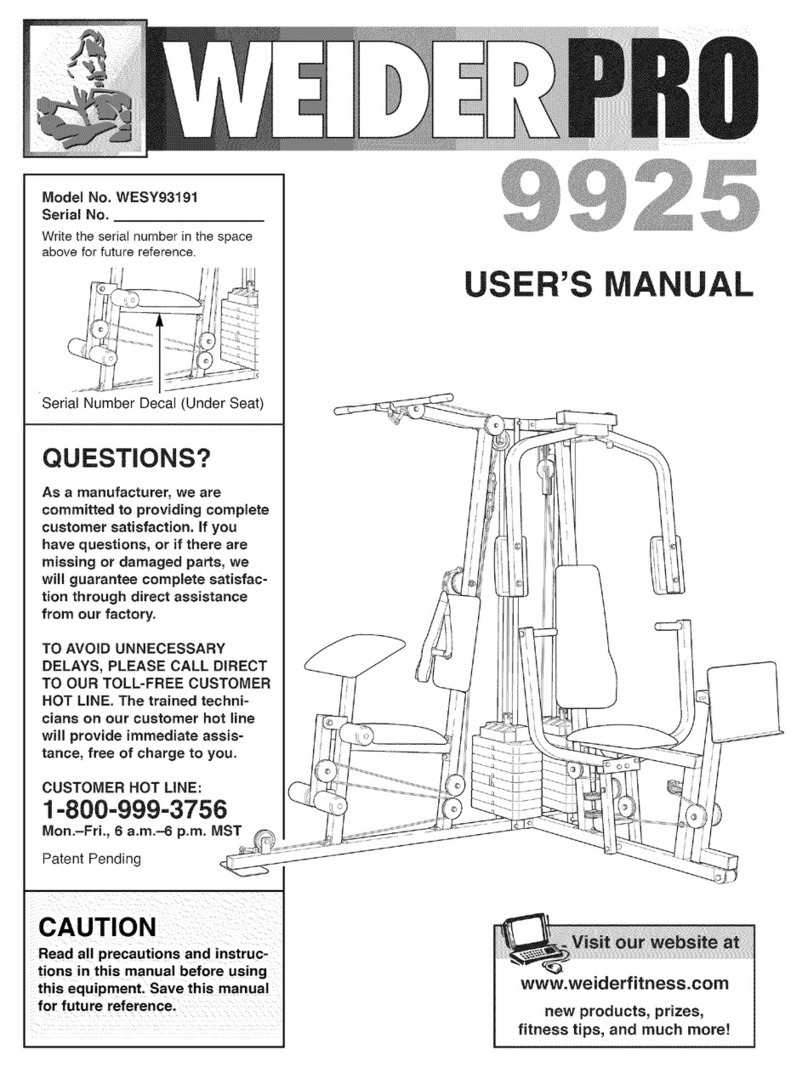
Classic™
SwallowStim
User Manual
Part#20752 October 2019 Rev. A
PRESCRIPTION INFORMATION:
Please read the following prescription
carefully before using your NMES unit.
If you have any questions regarding
this information, consult your health
care professional before using.
CAUTION: Federal Law restricts this device
to sale by, or on the order of, a practitioner
licensed by the state in which he or she
practices to use or order the use of the device.

23
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Safety First
Important Contraindications,
Warnings, Precautions and
Adverse Effects
Read carefully and understand fully before
using the Classic SwallowStim device
Contraindications:
This device should be used with caution on
patients with cardiac demand pacemakers.
Use of this device is contraindicated in patients
who are severely demented and exhibit non-stop
verbalization. Constant verbalization could
result in aspiration during trials of oral intake.
Use of this device is contraindicated in patients
with significant reflux due to use of a feeding
tube. Such patients are prone to repeated cases
of aspiration pneumonia, and the device has not
been studied in this population.
Use of this device is contraindicated in patients
with dysphagia due to drug toxicity. Patients
suffering from a drug toxicity could aspirate
during trials of oral intake.
This device should not be used when cancerous
lesions are present in the treatment area.
Table of Contents:
Safety First................................... 3
Warnings ....................................4
Precautions..................................5
Adverse Reactions ............................6
General Information..........................7
Getting Started ..............................11
Device Legend ..............................12
Display Legend..............................13
Simple Steps to Start Your First Therapy .......14
Electrode Instructional Guide..................18
Placements, Handling, and Type of Electrode
Configurations ..............................18
Technical Instruction Guide ...................24
Specification................................24
Batteries ...................................25
Miscellaneous...............................26
EMC Information ...........................27
Device Ownership Guide ......................28
Care and Handling of Your Device,
Troubleshooting and Device Warranty .........28
Warranty ...................................31
Notes ......................................32
Part Numbers for reorder ....................34
Declarations of Conformity...................35

45
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
1. The long-term effects of electrical
stimulation are unknown
2. Stimulation should not be applied
transthoracically in that the introduction
of electrical current into the heart may
cause cardiac arrhythmias
3. Stimulation should not be applied over
swollen, infected, or inflamed areas or skin
eruptions, e.g., phlebitis, thrombophlebi
tis, varicose veins, etc.
4. Do not apply stimulation in the presence
of electronic monitoring equipment (e.g.,
cardiac monitors, ECG alarms), which may
not operate properly when the electrical
stimulation device is in use
5. Do not apply stimulation when the patient
is in the bath or shower
6. Do not apply stimulation while the patient
is sleeping
7. Consult with the patient’s physician before
using this device because the device may
cause lethal rhythm disturbances to the
heart in susceptible individuals
8. Apply stimulation only to normal, intact,
clean, healthy skin
9. Do not apply stimulation across the patient’s
chest because the introduction of electrical
current into the chest may cause rhythm
disturbances to the patient’s heart, which
could be lethal
10. This unit must be used with the guidance
of a health care professional
11. Type BF equipment
12. Do not insert lead wires into a mains
power supply
13. Do not immerse unit into water or any
other substance
14. Do not use the Classic SwallowStim
Neuromuscular Electrical Stimulation
(NMES) unit in the presence of a
flammable anesthetic gas mixture and air
or with Oxygen or Nitrous Oxide
15. Never connect the device directly to a
battery charger or any other main
powered equipment
16. Patient Electrodes are for single patient
use only
17. Keep out of reach of children
18. Do not apply stimulation over, or in
proximity to, cancerous lesions
19. Do not apply stimulation while the patient
is driving, operating machinery, or during
any activity in which electrical stimulation
can put the patient at risk of injury
20. Stimulation should not be applied over
the carotid sinus nerves, particularly in
patients with a known sensitivity to the
carotid sinus reflex
Warnings
1. Precautions should be observed in the
presence of the following:
• When there is a tendency to hemorrhage
following acute trauma or fracture
• Following recent surgical procedures
when muscle contractions may disrupt
the healing process.
• Stimulation over the menstruating uterus
• Where sensory nerve damage is present
by a loss of normal skin sensation
2. Some patients may experience skin
irritation or hypersensitivity due to
the electrical stimulation or electrical
conductive medium. The irritation can
usually be reduced by use of an alternate
conductive medium, or alternate
electrode placement
3. Electrode placement and stimulation
settings should be based on the guidance
of the prescribing practitioner
4. Powered muscle stimulators should be
used only with the leads, electrodes, and
accessories recommended for use by the
manufacturer
5. Since the effects of stimulation of the brain
are unknown, stimulation should not be
applied across the head, and electrodes
should not be placed on opposite sides
of the head
6. Use this device only under the continued
supervision of a licensed practitioner
7. Use caution applying stimulation over the
patient’s neck because this could cause
severe muscle spasms resulting in closure of
the airway, difficulty in breathing, or adverse
effects on heart rhythm or blood pressure
8. Discontinue and do not increase the intensity
level if you feel discomfort during use
9. Stimulation should not be applied trans
cerebrally
10. Stimulation should be stopped if
discomfort is felt
11. The safety of electrical stimulation during
pregnancy has not been established
12. Patients with suspected or diagnosed
heart disease should follow precautions
recommended by their physicians
13. Patients with suspected or diagnosed
epilepsy should follow precautions
recommended by their physicians
14. Use caution when the patient has a
tendency to bleed internally, such as
following and injury or fracture
15. Use caution following recent surgical
procedures when stimulation may disrupt
the patient’s healing process
16. Use caution if stimulation is applied over
the menstruating or pregnant uterus
17. Use caution if stimulation is applied over
areas of skin that lack normal sensation
18. Keep this device out of reach of children
Precautions
* Long term effects of chronic Electronic Muscle Stimulation are unknown.
67
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Adverse Reactions
»Skin irritation and burns beneath the stimulation
electrodes have been reported with the use of powered
muscle stimulators.
»Patients may experience headache and other painful
sensations during or following the application of
electrical stimulation near the eyes and to the face.
»Patients should stop using the device and should
consult with their physicians if they experience
adverse reactions with the device.
»Patients may experience skin irritation and burns
beneath the stimulation electrodes applied to the skin
General Information Guide
Indicated for use:
Muscle re-education by application of external stimulation to the muscles necessary for
pharyngeal contraction.
*IMPORTANT ADVISORY*
CAUTION: Federal law restricts this device to sale by, or on the order of a licensed physician. This device should only be used under
medical supervision for adjunctive therapy and for the treatment of medical diseases and conditions.
To download the current version of this guide go to www.spectramedonline.com
* Made in the USA. FDA 510(k)120922. Designed in
California. Assembled and tested in FDA registered
facility in the USA. Some parts sourced globally.
89
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
General Information Guide
The Classic™is manufactured in the USA*
This manual is published by Spectramed, LLC. © All Rights Reserved
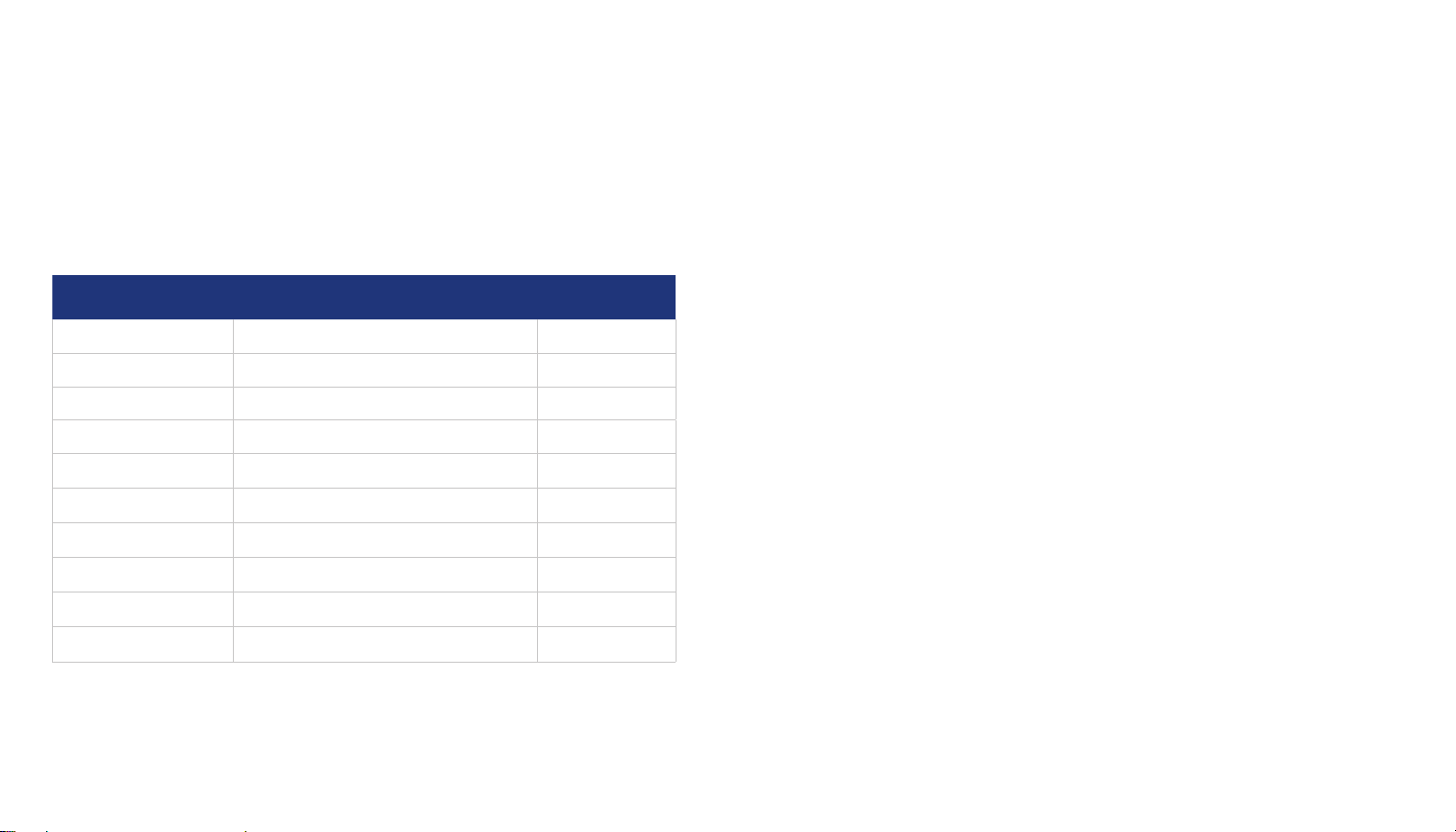
The Classic SwallowStim device conforms with the national and international medical device
standards and regulations described in the table below.
* Made in the USA. FDA 510(k)120922. Designed in California. Assembled and tested in FDA registered facility in the USA. Some parts
sourced globally.
STANDARD or
REGULATION NO.
NAME ISSUANCE DATE
IEC 60601-1 3 A1 general requirements 2012/08
IEC 60601-1-2 electromagnetic disturbances 2014/02
IEC 60601-1-6 3.1 usability 2013/10
IEC 60601-1-9 environmentally conscious design 2013/10
IEC 60601-1-11 home healthcare environment 2015/01
IEC 60601-2-10 nerve and muscle stimulators 2016/04
IEC 62304 1.1 software in a medical device 2016/06
ANSI/AAMI 62366 usability engineering 2015/02
ANSI/AAMI NS4:2013 transcutaneous elect. nerve stimulators 2013/03
ISO 14971:2012 risk management 2012/07
The Classic SwallowStim Instructions For Use (also called IFU) is comprised of instruction guides
on varied topics, Device features such as getting started, maintaining the device in good working
order, or if the need arises, troubleshooting and receiving warranty service.
The instruction guide covers:
A. Safety First - Contraindications, Warnings,
Precautions and Adverse Effects
B. General Information
C. Getting Started
D. Electrodes Instructional Guide
E. Special Features
F. Therapy Programs
G. Technical Information
H. Device Ownership
I. Clinical Information

10 11
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
* SAFETY AND ADVISORY NOTICES *
Read all instructions, warnings
and precautions (including these
notices) before using
this device in a therapy.
Protect this device from direct
exposure to the sun.
STOP — make sure you are
not subject to any of the
contraindications before using
this device in a therapy.
Protect this device from falling water.
Do not use this device in rain or in
the shower.
Do not use this device while under
the inuence of alcohol or drugs or
while intoxicated for any reason.
The safe operating temperature
range for this device is 15C (41F)
to 40C (104F).
This device is ONLY to be used by
and for the benet of the person
to whom it was prescribed.
The safe storage and transportation
temperature range for this device is
-25C (-13F) to 55C (131F).
Keep this device out of the reach
of children.
The safe atmospheric pressure
range for this device is 700hPa to
1013hPa or 9882 feet above sea
level to sea level.
Follow your physician’s instructions
as to which therapy presets to use,
electrode placement and suggested
amplitude levels.
The safe humidity operating
range for this device is 5% to 95%
relative humidity.
Familiarize yourself with the
features, benets and operation
of this device before use.
Dispose of batteries responsibly.
Search the Internet for information
about the safe disposal of batteries.
Getting Started
device
Part # 01GS25
028
CS1
:32
021 015
mA
CH1
mA
CH2
REMAIN
SEC
NMES
pin connect lead wires (2)
Part # 07RAP40
snap connect lead wires (2)
Part # 07MS36
manualcarrying case AA batteries (4)
case boot (optional; packaged separately)
15
40
-25
55
700 hPa
1013 hPa
5
95
General Information Guide
028
CS1
:32
021 015
mA
CH1
mA
CH2
REMAIN
SEC
NMES

12 13
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Getting Started
Classic SwallowStim — Device Legend
Display section [CS1 program]
Classic SwallowStim — Display Legend
Therapy Program Number
NMES Therapy
Channel 2 Indicator
Channel 2 Output Level
MilliAmp Indicator
NMES Contraction Indicator
Elapsed Time
Remaining Time
Seconds Indicator
Seconds Value
Minutes/Seconds Delimiter
The LCD is backlight illuminated for user convenience. The backlight only stays illuminated for
a few seconds to conserve battery power. During a therapy the backlight can be turned on by
pressing any button on the keypad with the exception of the power button which will pause the
therapy. When the device is off (not in a therapy, not just the display off) the backlight can be
turned on by pressing any of the keypad buttons.
028
CS1
:32
021 015
mA
CH1
mA
CH2
REMAIN
SEC
NMES
Display section: Therapy output level in mA
Ch1 (left) and Ch2 (right) output jacks
Display section: Battery and therapy duration status
Power button [turns device on and off / pauses therapy
+ Button (channels) [increases therapy output]
Clock button [access and set therapy duration]
- Button (channels) [decreases therapy output]
Star button [pause, restore and unlock]
888
CS1
:88
888 888
mA
CH1
mA
CH2
ELAPSED
REMAIN
SEC
PAUSED
NMES
Keypad Locked Graphic
Channel 1 Indicator
Channel 1 Output Level
Star Graphic
MilliAmp Indicator
NMES Contraction Indicator
“Paused” Message
Battery Status Graphic
Clock Graphic
Minutes Value

14 15
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
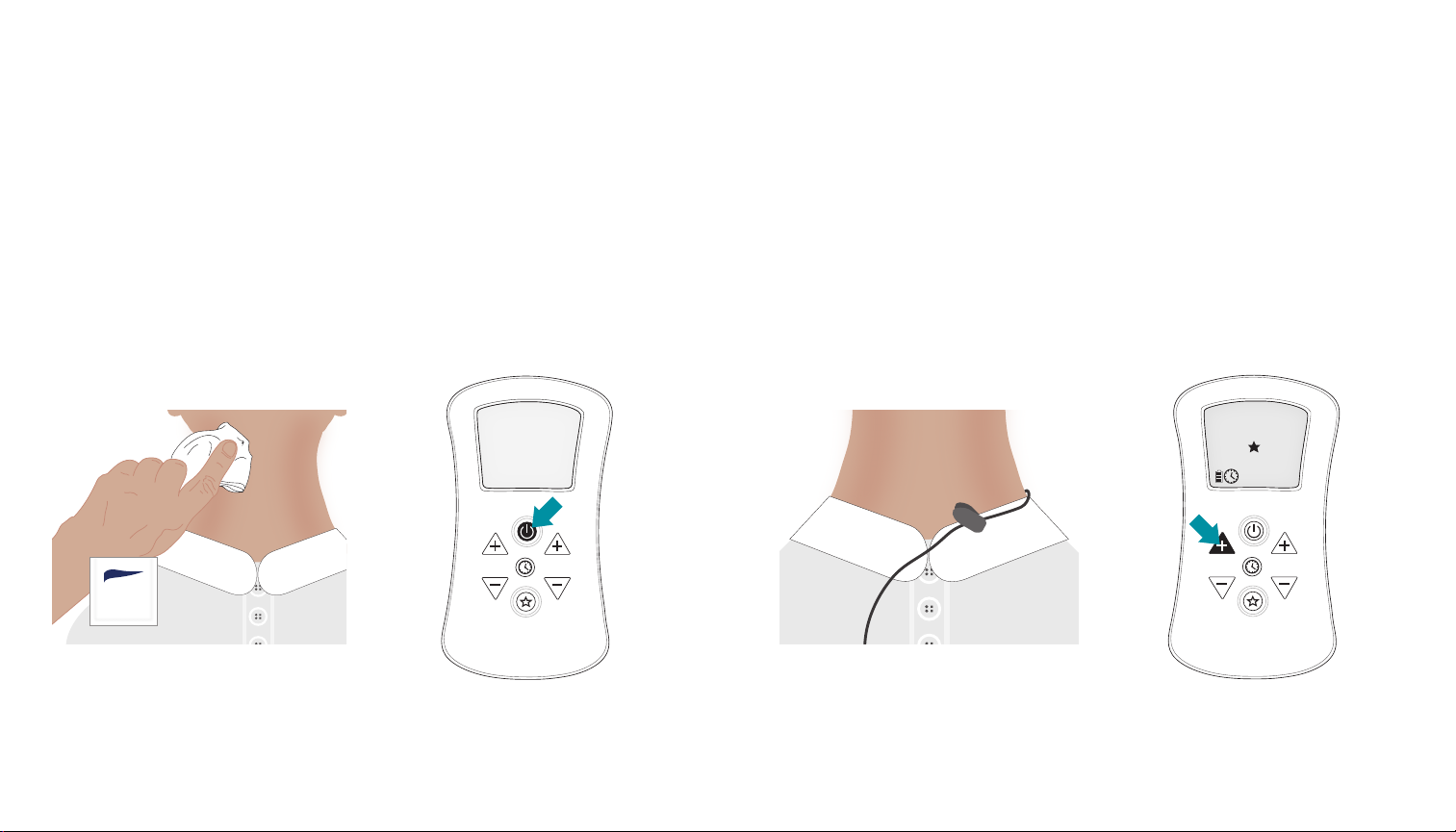
Simple Steps to Starting Your First Therapy
Step 1
Insert Batteries
Remove the battery door and insert (4) AA
batteries. Observe the battery’s polarity (+)
markings and the polarity (+) markings
inside the battery compartment.
Re-attach the battery door.
Step 2
Connect Lead Wires to Device
Connect each patient lead wire to the output
jacks at the top of the stimulation device.
Make sure the connectors are fully inserted.
All of the lead wires are interchangeable in
the two jacks
Step 3
Connect Lead Wires to Electrodes
Connect the patient lead wires to the electrodes
while they are on the electrode liner. Make
sure the pin connector is fully inserted into the
electrode receptacle. Any of the electrodes can
be connected to any of the lead wire pins.
Step 4
Determine Electrode Placement
Refer to Electrode Placement Section for
guidance on the correct arrangement of the
electrodes according to the type of therapy.
Correct electrode arrangement is vital to
receiving a safe and effective therapy.
[see pages 18-23]
Getting Started
16 17
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Step 5
Applying the Electrodes
Prior to applying the electrodes, properly
clean the skin around the treatment area with
soap and water. Dry the skin in the treatment
area thoroughly. Gently wipe area with skin
protectant barrier wipe. Do not use alcohol prep pads.
Remove the electrodes from the electrode
liner one at a time and apply to the skin in the
desired arrangement and location. Press the
electrodes to the skin to assure good contact.
Step 6
Power On the Device
Power on the device by pressing the Power
button. The device display and keypad will
illuminate.
Simple Steps to Starting Your First Therapy
Step 7
Attach
Attach lead wire clip to patient. Make certain
there is enough slack in the lead wire to allow
head movement.
Step 8
Increase Therapy Output
Press either of the “+” buttons to start the
therapy. The display will update to include the
therapy output indicators and the therapy clock
will begin counting down from 60 minutes. With
each “+” button press you will incrementally
increase the therapy output. If you press and
hold the “+” button you will increase the
therapy output at an increased rate.
000
CS1
:22
022 022
mA
CH1
mA
CH2
ELAPSED
REMAIN
SEC
NMES
Protective
Barrior
wipe
Getting Started

18 19
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Technical Information Guide
Classic SwallowStim Device — Specications
SPECIFICATIONS
Therapy Channels 2
Therapy Output Mode Neuromuscular Electrical Stimulation (NMES)
Preset Therapy Programs 1
Waveform Characteristics Square, Symmetrical, Bi-Phasic
Output Voltage Range
All values are VAC P2P
0-25 VAC P2P
Output Current Range
All values are mA P2P
0-50mA P2P @ 500 Ohm load
Each channel employs isolated intensity potentiometers.
Constant current control.
Pulse Width Range
Microseconds
300
Pulse Frequency Range in Hz 80
Internal Power Supply (4) AA Cells (1.5V) DO NOT USE LITHIUM CELLS>1.6V
Dimensions inches (mm) 2.6” x 5” x 1.1” (67 x 127 x 28)
Weight (grams) W/O battery 7.5 oz. (212)
Operating Conditions Temp 41F - 104F, Atmos. Press 700hPa - 1013hPa, Hum. 15% -
95%
Storage & Transport
Conditions
Temp -6F - 131F, Atmos. Press. 700hPa - 1013hPa, Hum. 5% - 95%
Classic SwallowStim Device — Batteries
Battery Size and Voltage
The battery size is AA (also known internationally
as LR6). The voltage varies depending on the
battery chemistry type but the typical voltage
range for individual cells is 1.2 VDC to 1.5VDC.
Battery Type
GOOD — The recommended battery type for
the Device is Alkaline or rechargeable Nickel
Metal Hydride (NiMH). Lithium Manganese
Dioxide batteries with a voltage of 1.5VDC are
an excellent choice but they are expensive.
BAD — NiCad rechargeable batteries are
not recommended due to their low capacity
and diminished performance with repeated
charge cycles. So-called “Super Heavy Duty”
(“SHD”) batteries are not recommended. The
chemistry in SHD batteries is zinc chloride
and they have an attractive up-front low-
cost but their extremely limited capacity and
premature exhaustion makes them one of the
most expensive choices to power the Device.
Avoid Lithium Iron Phosphate (also known as
LiPo) batteries with a voltage range of 3.0VDC
to 3.7VDC. The use of (4) LiPo batteries will
supply too high a voltage and permanently
damage the Device and void the warranty.
Battery Disposal
Alkaline batteries are safe to dispose of along
with household trash. In Oregon, USA, the
cells must be taken to a recycling center. Use
the Internet to research Oregon’s battery
disposal laws. NiMH rechargeable cells must
be disposed of according to State law in the
United States. Your State, Province, County or
Country may have different laws. Search the
Internet for battery disposal laws in your area.
Battery Polarity
Observe battery polarity when inserting
batteries into the Device. There are polarity
markings inside the battery compartment.
Additionally the Getting Started Guide
includes an illustration of the correct polarity
to observe. Visit www.spectramedonline.com
for a video of the correct method of installation
of the batteries.
Battery Service Life
There are many variables to consider when
estimating battery service life such as the
battery type, the freshness of the batteries
when placed into service, the quality and
capacity of the batteries, the output levels
of your therapies, the length of your therapy
durations and other factors. Considering the
variables it is impossible to estimate battery
service life.

20 21
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Technical Information Guide
Classic SwallowStim Device — Miscellaneous
Device Service Life
The Device Service Life is unlimited. The
Device Service Life is not the same as the
device Warranty. For Warranty information
please see the Device Ownership Guide.
Device Shelf Life
The Device Shelf Life is unlimited. The
shelf life of electrodes and batteries are
not associated with the Device shelf life.
Device Disposal
At the end of its service life the Device is small
enough to be disposed of with household trash.
Size and Type of Electrodes
Premium Spectramed dysphagia electrodes
such as the Guardian®brand are recommended
for use with the Device . Low quality or “value”
electrodes should be avoided due to their low
quality materials, poor therapeutic performance
and short service life.
There are many shapes and sizes of electrodes
available on the market. For safety reasons do
not use electrodes smaller than .5 Sq. In. (3.24
CM2) with NMES.
Classic SwallowStim Device — EMC Information
EMC - Electromagnetic Compatibility
This is the aggregate term for characteristics
of an electrical device that determine its
ability to function in an environment (such
as clinical, hospital, home health, outdoors,
airplanes etc.) with other electrical devices
without the Classic SwallowStim Device
interfering with their operation or without the
device being interfered with by other devices.
There are international standards that apply
to Electromagnetic Compatibility and devices
under test must meet or exceed the test
standards. The international standard is
IEC 60601-02-2016.
Electromagnetic Interference
The Classic SwallowStim has been tested in
the USA by a National Laboratory to determine
the electromagnetic radiation for the device.
Testing has confirmed that the Classic
SwallowStim does not emit any harmful
electromagnetic radiations.
Electromagnetic Immunity
The Classic SwallowStim has been tested in the
USA by a National Laboratory while in normal
operation, in the standard therapy program
and connected to a representative load. While
in operation many different electromagnetic
radiations bombarded the test device and in
all instances the device operated normally.
Static Charge Immunity
The Classic SwallowStim has been tested in the
USA by a National Laboratory while in normal
operation and exposed to static electricity
according to the International Standards.
In all Static Charge tests there was no
interruption to the device’s operation and
no damage to the device.
Magnetic Immunity
This final test is directed at the very rare
chance that the device would operate in an
environment of high magnetic fields. The device
passed all Magnetic Field Immunity tests.
22 23
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Cleaning Your Device
»Clean the device, as needed, by wiping
gently with a damp cloth and mild soap.
»Do not use abrasive cleaners or cleaners
containing solvents.
»Do not immerse the device in water or
other liquids. Do not splash water or
other liquids on the device.
Storage and Transportation
»Remove the batteries and place the device
and all accessories in the provided carrying
case or storage box.
»Refer to the General Information Guide
for the suggested range of storage and
transportation conditions.
»Protect the device from exposure to
water during storage and transportation.
»The device can safely be transported in
the passenger cabin or luggage
compartment of modern aircraft.
Customer Service
Contact the party you purchased the device
from or the organization that dispensed
the device to you. For questions related to
operating your device refer to the Instructional
Guide supplied with the device. If the guides
do not answer your questions please go
online to www.spectramedonline.com where
you will find articles that provide additional
information for many of the topics related to
the device operation.
Troubleshooting
Review the table on the next page for the
most common device complaints. As an
added troubleshooting resource visit
www.spectramedonline.com.
Device Ownership Guide
Care and Handling of Your Device,
Troubleshooting and Device Warranty
COMPLAINT POTENTIAL CAUSE/ POTENTIAL SOLUTION
Device does not power on 1. Remove and reinsert batteries carefully observing battery polarity
2. Insert fresh batteries
Stimulation is not felt 1. Conrm patient lead wires are fully inserted into the device
output jacks.
2. Conrm stimulation output levels are high enough to feel
stimulation.
3. Inspect patient lead wires for damage
Uncomfortable
stimulation
1. Decrease output level
2. Old electrodes/use fresh electrodes
“NO CON” message
appears when a therapy
is begun and the
amplitude is increased
1. “NO CON” message is intended to alert the user that a lead
wire(s) is not connected to the device or to the electrode
2. The lead wire could be damaged. Contact your dealer or
supplier and request a replacement lead wire.
Battery life is short 1. Use fresh alkaline or NiMH rechargeable batteries
2. Conrm the batteries are not “Super Heavy Duty” or NiCad type
3. Review the user guide battery life section to determine if your
battery life is consistent with expected battery life
Electrodes do not
adhere well
This is not a device issue. Electrodes are a consumable accessory
and subject to wearing out. Do not undergo a therapy with
electrodes that do not adhere well to your skin. Use fresh electrodes
to ensure a safe and effective therapy and minimize adverse events.
Display illumination is dim Insert fresh batteries. If problems persist le a warranty claim
with Warrantor.
Output is intermittent Test lead wires with integrated lead wire tester (see Special Features
Guide). If lead wire tester determines there is a short in the lead
wire, request a replacement lead wire from your dealer or supplier.

24 25
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Device Ownership Guide
Care and Handling of Your Device,
Troubleshooting and Device Warranty
COMPLAINT POTENTIAL CAUSE / POTENTIAL SOLUTION
Output stops abruptly
and prematurely
1. Batteries are exhausted.
2. Lead wires have become disconnected from device or electrodes.
3. Devise may be in PAUSE state.
Device is powered off
but stimulation is felt
Try to duplicate the problem.
If the problem persists le a warranty claim.
After unpausing output
is zero
Cycle the device on and off.
If the problem persists le a warranty claim.
Display does not stay
illuminated
This is normal. To conserve battery power the display illumination
ends after ve seconds of keypad inactivity.
Some of the display
characters are not
fully formed
Cycle the device on and off.
If the problem persists le a warranty claim.
Device is stuck on one
of the programs when
I turn it on
This is normal. The Classic SwallowStim is a single program device.
Classic SwallowStim Device — Warranty
Spectramed, LLC, (Warrantor) warrants its Electrotherapy Device(s) against any defect
in materials and/ or workmanship for a period of one year from the date the Device is
dispensed to the user/patient or 15 months from the date of original sale whichever is
longer (Warranty Period) and promises to repair or replace, at its sole discretion, any of
its Devices that fail due to defects in materials and/or workmanship. The serial number
of the Device is automatically registered at the time of initial sale for establishing the
Warranty Period start date and no additional warranty registration is required.
This Warranty does not cover a Device that has been damaged by accident, shipment,
dropping, exposure to water, misuse, abuse, neglect, improper servicing, or from any
cause not arising out of defect in original materials and/or workmanship. This Warranty
does not cover devices that have been damaged by the use of Lithium 3.7 volt AA
batteries. This Warranty does not cover a Device that has been altered in any way or
repaired by any personnel or entity other than the original manufacturer. This Warranty
does not cover Devices that have been entered into rental service. This Warranty does
not cover accessories such as batteries, lead wires, electrodes, belt clips or carrying case.
To make a Warranty Claim, contact the Warranty Service Processor (either the party
you purchased the Device from or the entity that dispensed the Device to you).
DO NOT CONTACT Warrantor. Locate the Device serial number located on the back
of the Device. Provide the Device serial number to the Warranty Service Processor so
that it can be verified for Warranty coverage. Follow the instructions of the Warranty
Service Processor.
The resolution of any Warranty Claim will be at the sole discretion of Spectramed, LLC
and is limited to repair or replacement of the Device. Spectramed, LLC assumes no
responsibility for incidental, direct, consequential or compensatory loss or damage
related to an accepted Warranty Claim. No refunds on the purchase of a Device will be
offered by Warrantor. An accepted Warranty Claim whether the Device is repaired or
replaced does not extend or alter in any way the original Device’s Warranty Period or
change the original Device’s Warranty terms.
Manufactured in the USA for
Spectramed, LLC.
www.spectramedonline.com

26 27
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Notes

28 29
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Notes
30 31
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
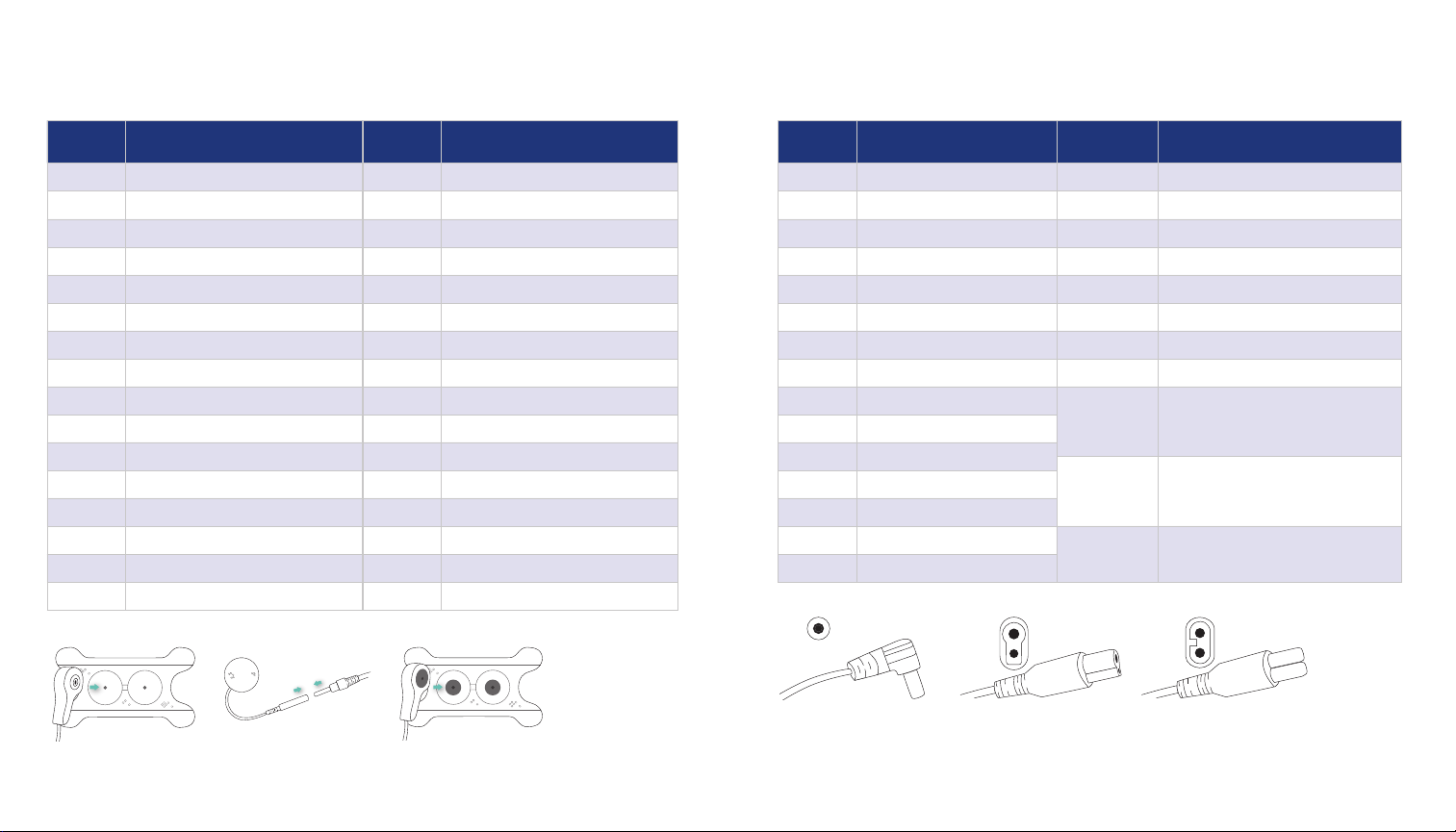
Classic SwallowStim Device — Part numbers for re-order
PART
NUMBER DESCRIPTION
*TH1183BC-10 10 pks .875” Magnetic Connection w/buttery
*TH1183BC-30 30 pks .875” Magnetic Connection w/buttery
*TH1183BC-50 50 pks .875” Magnetic Connection w/buttery
*TH1183BC-100 100 pks .875” Magnetic Connection w/buttery
*TH1183BCS-10 10 pks 1” Magnetic Connection
*TH1183BCS-30 30 pks 1” Magnetic Connection
*TH1183BCS-50 50 pks 1” Magnetic Connection
*TH1183BCS-100 100 pks 1” Magnetic Connection
07TH5101 51” Lead connects exclusively with TH1183
series electrodes
Lead wire compatible with Guardian Unity and
Ampcare devices
07TH5102 51” Lead connects exclusively with TH1183
series electrodes
Lead wire compatible with VitalStim and
eSwallow devices
07TH5103 51” Lead connects exclusively with TH1183
series electrodes
Lead wire compatible with VitalStim Plus device
PART
NUMBER DESCRIPTION
05A163-30 30 pks .875 wired electrodes
05A163-50 50 pks .875 wired electrodes
05A163-100 100 pks .875 wired electrodes
05A164-10 10 pks 1” mini-snap electrodes
05A164-30 30 pks 1” mini-snap electrodes
05A164-50 50 pks 1” mini-snap electrodes
05A164-100 100 pks 1” mini-snap electrodes
05A165-10 10 pks .875” wired buttery
05A165-30 30 pks .875” wired buttery
05A165-50 50 pks .875” wired buttery
05A165-100 100 pks .875” wired buttery
05A170-10 10 pks .68” x .87” Pediatric wired oval
05A170-30 30 pks .68” x .87” Pediatric wired oval
05A170-50 50 pks .68” x .87” Pediatric wired oval
05A170-100 100 pks .68” x .87” Pediatric wired oval
*07TH5101 07TH5102 07TH5103
PART
NUMBER DESCRIPTION
05A150-10 10 pks .6875” mini-snap electrodes w/buttery
05A150-30 30 pks .6875” mini-snap electrodes w/buttery
05A150-50 50 pks .6875” mini-snap electrodes w/buttery
05A150-100 100 pks .6875” mini-snap electrodes w/buttery
05A151-10 10 pks .875” mini-snap electrodes w/buttery
05A151-30 30 pks .875” mini-snap electrodes w/buttery
05A151-50 50 pks .875” mini-snap electrodes w/buttery
05A151-100 100 pks .875” mini-snap electrodes w/buttery
05A152-10 10 pks .875” mini-snap electrodes
05A152-30 30 pks .875” mini-snap electrodes
05A152-50 50 pks .875” mini-snap electrodes
05A152-100 100 pks .875” mini-snap electrodes
05A154-10 10 pks .875” mini-snap electrodes Hydroblue
05A154-30 30 pks .875” mini-snap electrodes Hydroblue
05A154-50 50 pks .875” mini-snap electrodes Hydroblue
05A154-100 100 pks .875” mini-snap electrodes Hydroblue
PART
NUMBER DESCRIPTION
05A156-10 10 pks 1.0” wired electrodes Hydroblue
05A156-30 30 pks 1.0” wired electrodes Hydroblue
05A156-50 50 pks 1.0” wired electrodes Hydroblue
05A156-100 100 pks 1.0” wired electrodes Hydroblue
05A158-10 10 pks .875” wired electrodes Hydroblue
05A158-30 30 pks .875” wired electrodes Hydroblue
05A158-50 50 pks .875” wired electrodes Hydroblue
05A158-100 100 pks .875” wired electrodes Hydroblue
05A159-10 10 pks 1” x 1.25” wired electrodes Hydroblue
05A159-30 30 pks 1” x 1.25” wired electrodes Hydroblue
05A159-50 50 pks 1” x 1.25” wired electrodes Hydroblue
05A159-100 100 pks 1” x 1.25” wired electrodes Hydroblue
05A160-10 10 pks 1.0” wired electrodes
05A160-30 30 pks 1.0” wired electrodes
05A160-50 50 pks 1.0” wired electrodes
05A160-100 100 pks 1.0” wired electrodes
Snap connect Pin connect Magnet connect
32 33
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
GUIDANCE AND MANUFACTURER’S DECLARATION — ELECTROMAGNETIC EMISSIONS
The Classic SwallowStim electrotherapy system is intended for use in the electromagnetic environment dened
below. The customer or the user of the Classic SwallowStim system should assure that it is used in such an
environment.
EMISSIONS TESTS COMPLIANCE ELECTROMAGNETIC ENVIRONMENT - GUIDANCE
RF emissions
CISPR 11
Group 1 The Classic SwallowStim electrotherapy system uses RF energy,
only, for it internal function. Therefore, its RF emissions are very
low and are not likely to cause any interference with near by
electronic equipment.
RF emissions
CISPR 11
Class B The Classic SwallowStim electrotherapy system is suitable for
use
in all establishments, and those directly connected to the public
low voltage power system network that supplies buildings used
for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Not applicable -
battery powered
Voltage uctuation
IEC 61000-3-3
Not applicable -
battery powered
Electromagnetic Compatibility EMC Tables
GUIDANCE AND MANUFACTURER’S DECLARATION — ELECTROMAGNETIC IMMUNITY
The Classic SwallowStim electrotherapy system is intended for use in the electromagnetic environment specied
below. The customer or the user of the Classic SwallowStim system should assure that it is used in such an
environment.
IMMUNITY
TEST IEC 60601
TEST LEVEL COMPLIANCE
LEVEL ELECTROMAGNETIC ENVIRONMENT
- GUIDANCE
Electrostatic
discharge (ESD)
IEC 61000-4-2
+6kV contact
+8kV air
+6kV contact
+8kV air
Risk Assessment on the Classic SwallowStim
electrotherapy system indicates the
compliance levels claimed are acceptable
when EDS-precautionary measures are taken.
Electrical fast
transient/burst
IEC 61000-4-4
+2kV for power
supply lines
+1kV for input/
output lines
Not applicable
- battery powered
Not applicable -
signal lines less
then 3 meters
Main power quality should be that of a
typical commercial or hospital environment.
Surge
IEC 61000-4-5
+1kV differential
mode (line to line)
+2kV common mode
(line to ground)
Not applicable -
battery powered
Main power quality should be that of a
typical commercial or hospital environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5% UT(>95% dip
in UT) for 0,5 cycle
40% UT(60% dip in
UT) for 5 cycles
70% UT(30% dip in
UT) for 25 cycles
<5% UT (>95% dip
in UT) for 5 sec.
Not applicable -
battery powered
Main power quality should be that of a typical
commercial or hospital environment. If the
user of the Classic SwallowStim electrotherapy
system requires continued operation during
power mains interruptions, it is recommended
that the Classic SwallowStim electrotherapy
system be powered from an uninterrupted
power supply.
Power frequency
(50/60Hz)
magnetic eld
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic elds should be at
levels characteristic of a typical location in a
typical commercial or hospital environment.
NOTE: UT is the AC mains voltage prior to application of the test level.
Declarations of Conformity

34 35
Draft Part# 20752 Rev. Print Date xx/xx/xxxx Draft Part# 20752 Rev. Print Date xx/xx/xxxx
Electromagnetic Compatibility EMC Tables
RECOMMENDED SEPARATION DISTANCES BETWEEN PORTABLE AND MOBILE RF
COMMUNICATIONS EQUIPMENT AND THE CLASSIC SWALLOWSTIM ELECTROTHERAPY
SYSTEM
The Classic SwallowStim electrotherapy system is intended for use in an electromagnetic environment in which
radiated RF disturbance are controlled. The customer or the user of the Classic SwallowStim can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile RF communication
equipment (transmitters) and the Classic SwallowStim electrotherapy system as recommended below, according
to the maximum output power of the communications equipment.
RATED MAXIMUM
OUTPUT POWER
OF TRANSMITTER
P(W)
SEPARATION DISTANCE ACCORDING TO FREQUENCY OF TRANSMITTER
d(m)
150 kHz to 80 MHz
d=[3,5]√P
V1
(where V1=3V)
80 MHz to 800 MHz
d=[3,5]√P
E1
(where E1=3V/m)
800 MHz to 2,5 GHz
d=[7]√P
E1
(where E1=3V/m)
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
11,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance (d) in meters (m) can be estimated
using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from
structures, objects and people.
GUIDANCE AND MANUFACTURER’S DECLARATION - ELECTROMAGNETIC IMMUNITY
The Classic SwallowStim electrotherapy system is intended for use in the electromagnetic environment dened
below. The customer or the user of this electrotherapy system should assure that it is used in such an environment.
IMMUNITY
TEST IEC 60601
TEST LEVEL COMPLIANCE
LEVEL ELECTROMAGNETIC ENVIRONMENT -
GUIDANCE
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80MHz
3 V/m
80 MHz to 2,5 GHz
[V1] V, where V1=3V
[E1] V/m, where E1=3V/m
Portable and mobile RF communications
equipment should be used no closer to any
part of the Classic SwallowStim electrotherapy
system, including cables, than the recommended
separation distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance
d=[3,5]√P
V1
d=[3,5]√P 80 MHz to 800 MHz
E1
d=[7]√P 800MHz to 2,5 GHz
E1
where P is the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and d is the
recommended separation distance in meters (m).
Field strengths from xed RF transmitters, as
determined by an electromagnetic site survey,a
should be less than the compliance level in each
frequency range.b
Interference may occur in the vicinity of
equipment marked with the following symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from structures,
objects and people.
a Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and
FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to xed RF
transmitters, an electromagnetic site survey should be considered. If the measured eld strength in the location in which the Classic SwallowStim
electrotherapy system is used exceeds the applicable RF compliance level above, the Classic SwallowStim should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Classic SwallowStim
electrotherapy system.
b Over the frequency range 150 MHz to 80 MHz, eld strengths should be less than [V1] V/m

1601 Eastgate Pkwy
Columbus, Ohio 43230
1-800-643-1917
www.spectramedonline.com
This manual suits for next models
1
Table of contents
Other Spectramed Fitness Equipment manuals
Popular Fitness Equipment manuals by other brands

K-SPORT
K-SPORT KSH004/SK Assembly instruction
X Training Equipment
X Training Equipment COMPETITION PULL-UP RIG Assembly guide
Balanced Body
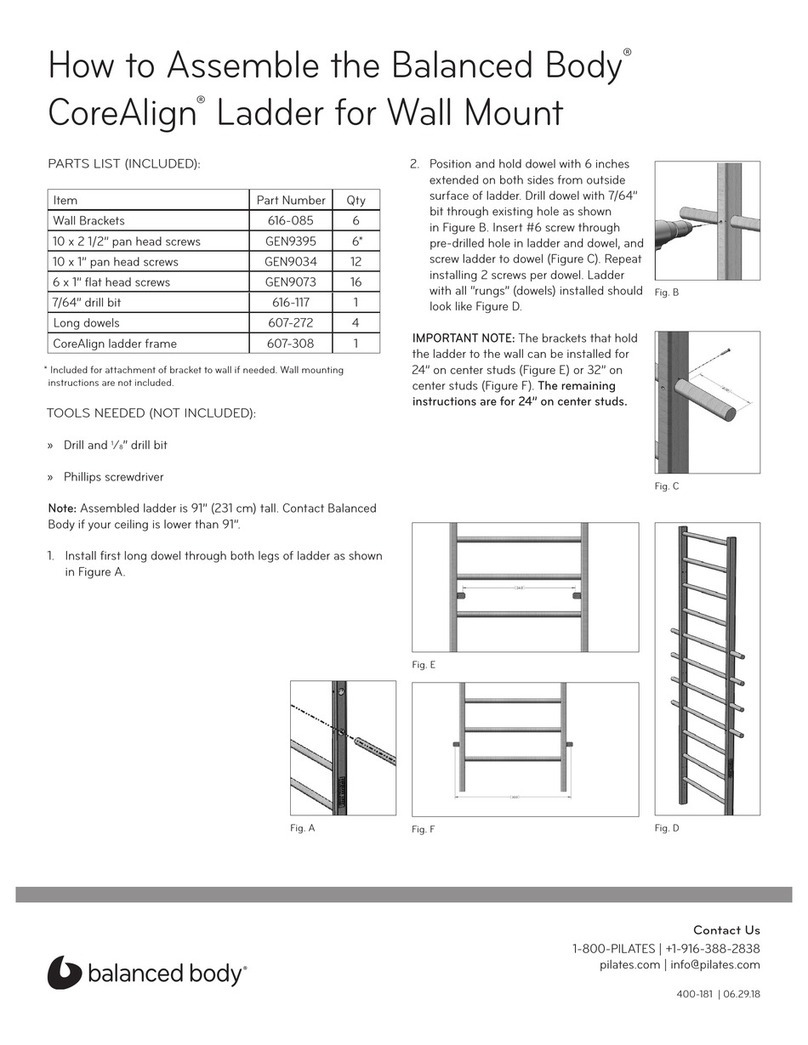
Balanced Body CoreAlign 616-085 Assemble instructions

Taurus
Taurus TF-SR7613 operating instructions

Matrix
Matrix MAGNUM SERIES quick start guide

SportsArt Fitness
SportsArt Fitness DF-102 manual