Storz E-BOX User manual

Instructions for use
E-BOX
TP012
en

Copyright ©
All product illustrations, product descriptions, and texts are the intellectual property of
KARLSTORZSE&Co.KG.
Their use and reproduction by third parties require the express approval of
KARLSTORZSE&Co.KG.
All rights reserved.
03-2021

Table of contents
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 3
Table of contents
1 General information.......................................................................................................................................4
1.1 Read the instructions for use.................................................................................................................... 4
1.2 Read the instructions for use of combinable products............................................................................. 4
1.3 Scope........................................................................................................................................................4
1.4 Initial commissioning ................................................................................................................................4
2 Normal use....................................................................................................................................................5
2.1 Intended use .............................................................................................................................................5
2.2 Indications.................................................................................................................................................5
2.3 Contraindications......................................................................................................................................5
2.4 Target user populations ............................................................................................................................ 5
2.5 Patient population.....................................................................................................................................5
3 Safety ............................................................................................................................................................6
3.1 Serious incidents ......................................................................................................................................6
3.2 Unsterile product ......................................................................................................................................6
3.3 Damaged products ...................................................................................................................................6
4 Product description ......................................................................................................................................7
4.1 Product overview ......................................................................................................................................7
4.2 Possible combinations..............................................................................................................................7
4.3 Technical data...........................................................................................................................................8
4.4 Ambient conditions ...................................................................................................................................8
4.5 Symbols on the packaging ....................................................................................................................... 9
5 Preparation..................................................................................................................................................10
5.1 Unpacking the product ...........................................................................................................................10
5.2 Testing the product.................................................................................................................................10
5.3 Connecting the product ..........................................................................................................................10
6 Disassembly................................................................................................................................................12
6.1 Disassembling the product ..................................................................................................................... 12
7 Maintenance, servicing, repairs, and disposal............................................................................................13
7.1 Repairing the product .............................................................................................................................13
7.2 Safety inspection in accordance with IEC 62353 ................................................................................... 13
7.3 Disposing of the product ........................................................................................................................13
8 Electromagnetic compatibility.....................................................................................................................14
8.1 General notes on the operating environment .........................................................................................14
8.2 Table 1 – Compliance level for immunity tests ....................................................................................... 14
8.3 Table 2 – Test levels for proximity fields from HF wireless communications equipment ....................... 16
8.4 Table 3 – Test levels for radiated and conducted immunity tests .......................................................... 17
8.5 Table 4 – Emission class and group .......................................................................................................18
8.6 Table 5 – Recommended separation distances between portable and mobile HF communications
equipment and the product ....................................................................................................................18
9 Subsidiaries.................................................................................................................................................20

General information
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 4
1 General information
1.1 Read the instructions for use
If the instructions for use are not followed, patients, users, and third parties may be injured or
the product may be damaged.
Read the instructions for use carefully and follow all the safety notes and warnings.
Keep the instructions for use clearly visible next to the product.
1.2 Read the instructions for use of combinable products
If the instructions for use of combinable products are not followed, patients, users, and third
parties may be injured or the product may be damaged.
Read the instructions for use of the combinable products carefully and follow all the safety
notes and warnings.
1.3 Scope
These instructions for use are valid for the following products:
E-BOX TP012
1.4 Initial commissioning
This product must be reprocessed before being used for the first time.

Normal use
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 5
2 Normal use
2.1 Intended use
The E-BOX is used for processing and transferring data from an endoscope to KARL STORZ
camera control units. The E-BOX has no direct contact with the human body.
2.2 Indications
The indication is defined by the device that is controlled using the additional operating
elements or for which the additional accessories are used.
2.3 Contraindications
The contraindication is defined by the device that is controlled using the additional operating
elements.
2.4 Target user populations
The medical device may only be used by doctors and medical assistants with a relevant
specialist qualification.
2.5 Patient population
There are no restrictions in terms of patient groups for this product.

Safety
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 6
3 Safety
3.1 Serious incidents
According to the Medical Device Regulation (MDR), a “serious incident” includes incidents that
directly or indirectly had, could have had, or could have any of the following consequences
(MDR, Art.2, No.65[1]):
– Death of a patient, user, or another person
– Temporary or permanent serious deterioration in the medical condition of a patient, user,
or another person
– A serious threat to public health
The manufacturer and appropriate authority must be notified of all serious incidents.
3.2 Unsterile product
The product is not sterile when delivered. The use of non-sterile products poses a risk of
infection for patients, users, and third parties.
Reprocess the product in line with the reprocessing instructions before initial use and
every subsequent use.
3.3 Damaged products
Damaged products can result in injury to patients, users, or third parties.
Before each use, check all components of the product for damage.
Do not use damaged products.
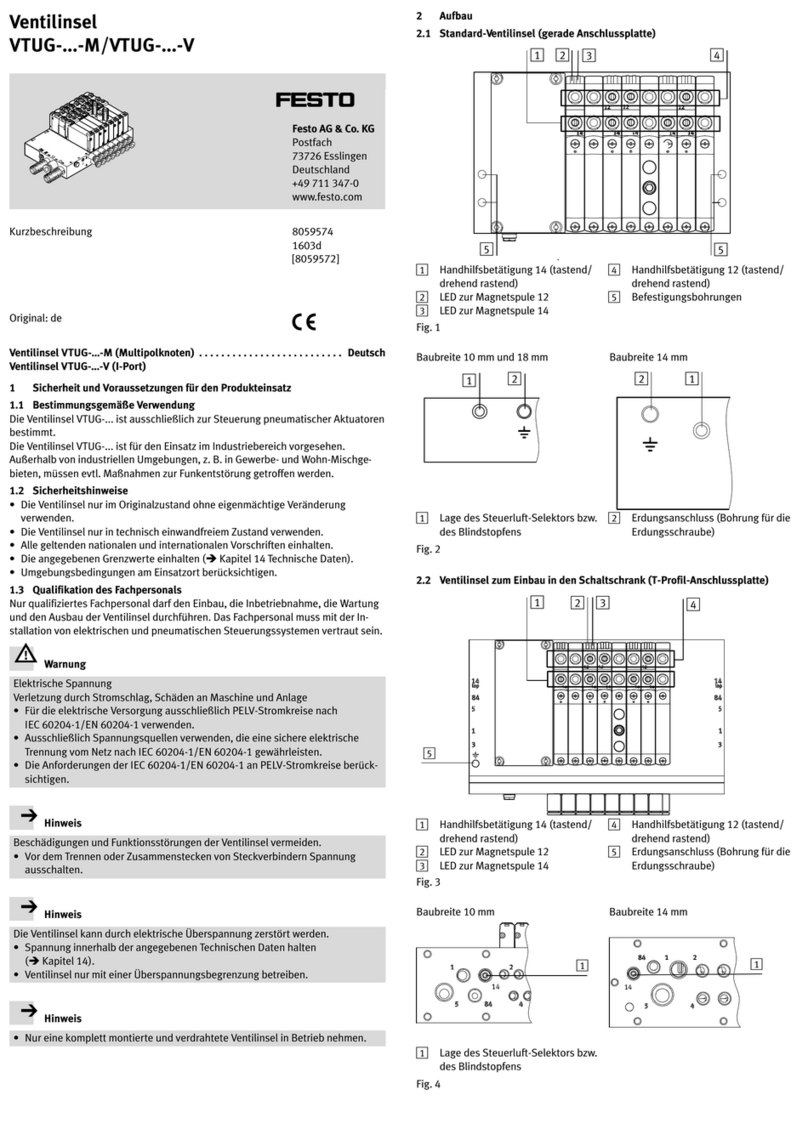
Product description
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 7
4 Product description
4.1 Product overview
4
1
2
3
5
6
1 Flexible single-use videoendoscope
2 E-BOX
3 Socket for flexible single-use videoendoscope
4 Indicator light
5 C-MAC monitor
6 Connecting cable for CCU
4.2 Possible combinations
The E-BOX TP012 is only suitable for use with the following KARL STORZ products:
Instruments Camera control units
E-BOX
TP012
KARL STORZ single-use videoen-
doscopes 091xxx
C-MAC® monitor 8403ZX (from soft-
ware version 704v302)
C-HUB® II 20290320 (from software
version 1.06)

Product description
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 8
4.3 Technical data
E-BOX TP012
Housing length 175mm
Cable length 200mm
Width 30mm
Depth 24mm
Weight Approx. 93g
Classification Class 1
Input voltage 5V
Power input 950mW
LED status display Green: ready for use
4.4 Ambient conditions
NOTICE
Damage due to ingress of liquid!
Liquid ingress into the product can cause a short-circuit which would damage the product.
Do not store any liquids on, above, or close to the product.
Do not spray the product directly during disinfection.
In the event of ingress of liquid into the product, switch off the product, disconnect it
from the power supply, and allow it to dry completely.
Storage and transport
Ambient temperature - 10° ... + 60°C
Relative humidity 5% ... 95%
Atmospheric pressure 700 ... 1080hPa
Application
Ambient temperature 0° ... 40°C
Relative humidity 30% ... 70%
Atmospheric pressure 700 ... 1080hPa

Product description
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 9
4.5 Symbols on the packaging
Symbol Meaning
Manufacturer
Date of manufacture
Medical device
Article no.
Serial number
Number of products in the product packaging
Unsterile
Temperature limit
Humidity limit
Air pressure limit
Keep dry
Consult instructions for use
Federal (USA) law restricts this device to sale by or on the order of a
physician.
CE conformity mark
With this mark, the manufacturer declares the compliance of the prod-
ucts with the applicable regulation (EU) 2017/745. A code number after
the CE mark indicates the responsible notified body.

Preparation
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 10
5 Preparation
5.1 Unpacking the product
1. Carefully remove the product and accessories from the packaging.
2. Check the delivery for missing items and evidence of shipping damage.
3. In the case of damage, hidden defects, and short deliveries, document their nature and
extent and contact the manufacturer or supplier immediately.
5.2 Testing the product
Check that the product is functioning properly and is in good working order.
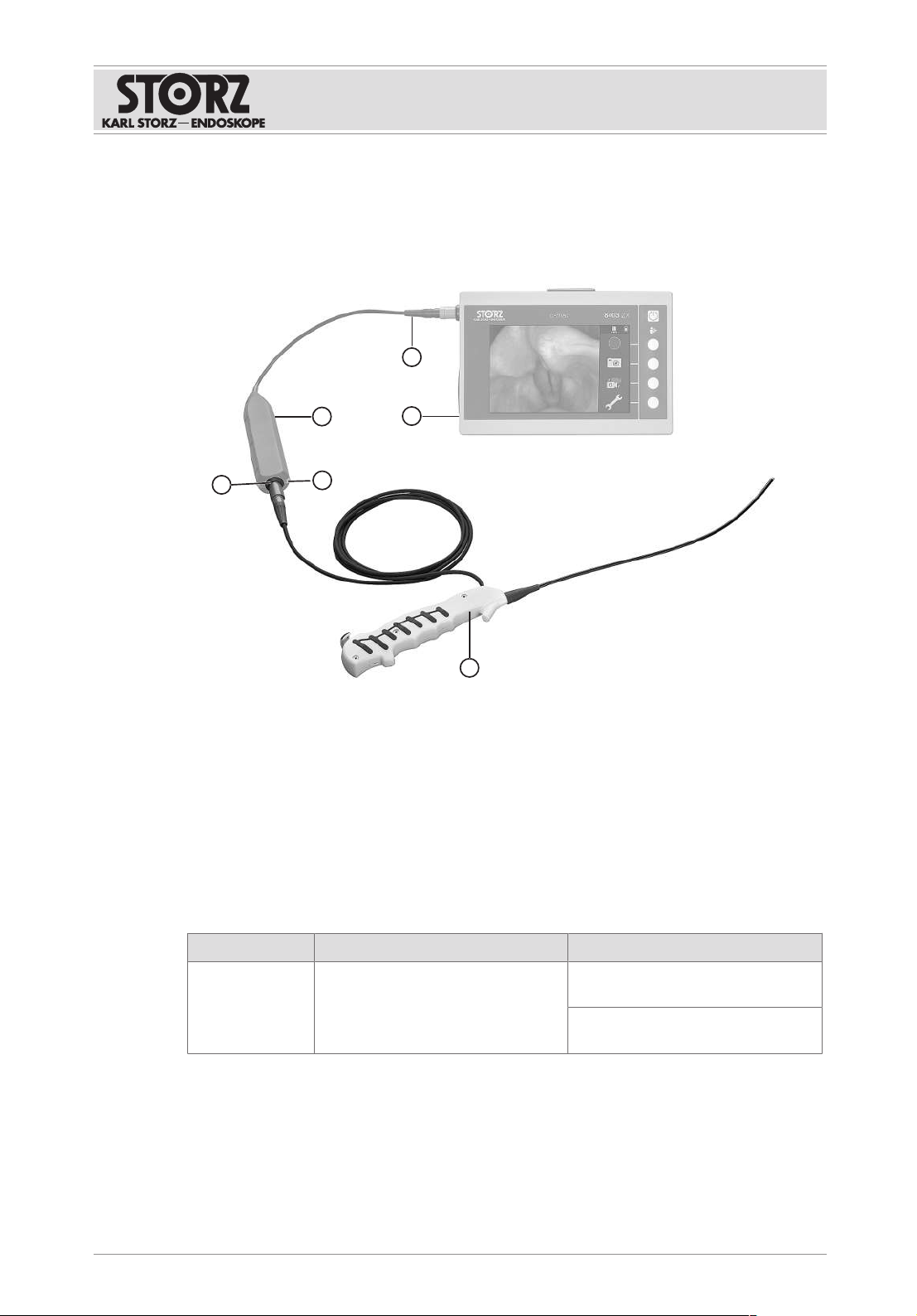
5.3 Connecting the product
NOTICE
Risk of product damage due to unsuitable accessories!
Using the plug-in adapter for the C-MAC® 8401XA in combination with the E-BOX can cause
damage to the product or the connected instrument.
Do not use the E-BOX in combination with the plug-in adapter for the C-MAC® 8401XA.
1. Connect the connecting cable for the flexible single-use videoendoscope to the E-BOX
for X-LINK socket.
2. Connect the connecting cable for the E-BOX for X-LINK to the corresponding port on the
camera control unit (CCU).

Preparation
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 11
3. Connect the CCU to the power supply and switch on at the power switch if necessary.
ðThe LED indicator light on the E-BOX lights up green.
ðThe E-BOX is ready for operation. A live image appears on the monitor.
If the LED indicator light does not light up green, this indicates a defect. Should this
occur, contact KARLSTORZ Service.
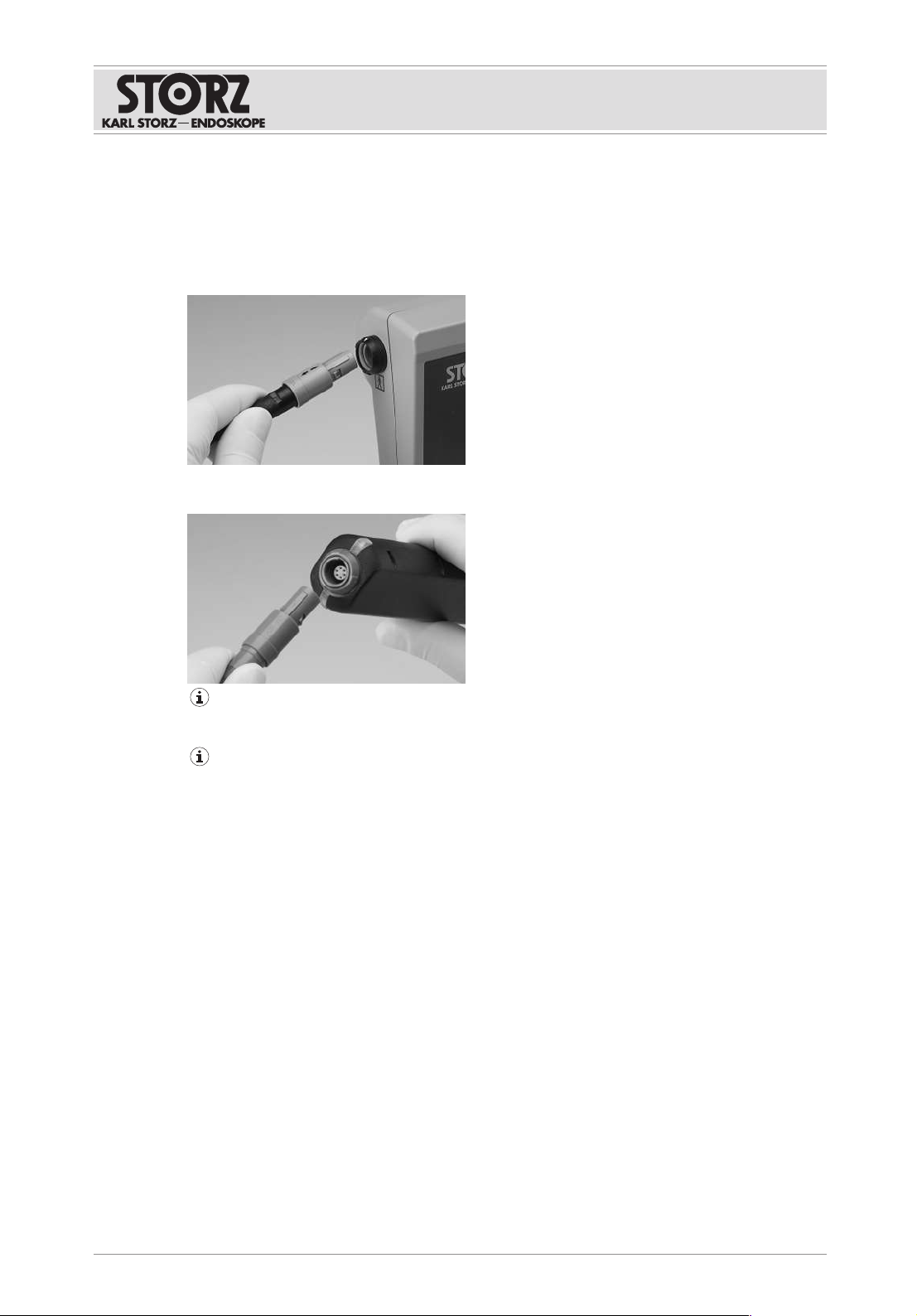
Disassembly
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 12
6 Disassembly
6.1 Disassembling the product
1. Unplug the E-BOX connector from the C-MAC monitor port or from the port on the C-
HUB II.
2. Disconnect the connecting cable for the flexible single-use videoendoscope from the E-
BOX socket.
To remove the cable from the C-MAC monitor and the E-BOX, firstly gently push in the
plug so that it can then be easily removed.
Do not dispose of the E-BOX after use. The E-BOX can be reused after each wipe-down
disinfection.

Maintenance, servicing, repairs, and disposal
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 13
7 Maintenance, servicing, repairs, and disposal
7.1 Repairing the product
Repair work may only be performed by KARLSTORZ or by a company authorized by
KARLSTORZ. The interventions described in this instruction manual are exempt from this rule.
Please contact your local KARLSTORZ subsidiary or authorized dealer (see the list of
subsidiaries).
Contaminated devices may not be shipped. To prevent contact infections and airborne
infections, products must first be decontaminated. KARLSTORZ reserves the right to send
back contaminated products.
7.2 Safety inspection in accordance with IEC 62353
WARNING
Risk of injury due to product deficiencies!
Patients, users, and third parties may be injured as a result of deficiencies with the product and
accessories.
Shut down the product.
Have the deficiencies repaired by persons authorized by KARLSTORZ.
Regardless of the national accident prevention regulations and testing intervals for medical
devices, for this device safety checks must be performed as repeat inspections according to
IEC62353 and recorded by a qualified electrician at least once a year. Detailed specifications
regarding the scope and execution of the safety inspection can be found in the service manual.
7.3 Disposing of the product
For disposal, the following measures are necessary:
1. Decontaminate the products prior to disposal.
2. Country-specific national laws and regulations must be observed.

Electromagnetic compatibility
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 14
8 Electromagnetic compatibility
8.1 General notes on the operating environment
The product is suitable for use in professional healthcare settings. Professional healthcare
facilities include physician offices, dental offices, limited care facilities, freestanding surgical
centers, freestanding birth centers, multiple treatment facilities, hospitals (emergency rooms,
patient rooms, intensive care, surgical rooms, outside the HF-shielded room of an ME system
for MRT).
The emission characteristics of this product make it suitable for use in professional
healthcare facilities as well as in a residential environment (CISPR 11 Class B). This
product offers adequate protection to radio communication service. In the rare event of
interference to the radio transmission operation, the user might need to take mitigation
measures, such as relocating or re-orienting the product.
Portable HF communications equipment (including peripherals such as antenna cables
and external antennas) should be used no closer than 30cm to any part of the device,
including cables specified by the manufacturer. Otherwise, performance may be
impaired.
The emission characteristics of this product make it suitable for use in professional
healthcare facilities as well as in a residential environment (CISPR 11 Class B). This
product offers adequate protection to radio communication service. In the rare event of
interference to the radio transmission operation, the user might need to take mitigation
measures, such as relocating or re-orienting the product.
The use of cables other than those specified in the instructions for use may result in
increased emissions or decreased immunity of the . The cables listed have been shown
to comply with the requirements of EN/IEC IEC 60601-1-2. When using other cables, the
operator is responsible for checking that they comply with IEC 60601—1-2:2014.
WARNING: The use of this device next to or with other devices should be avoided, as this can
result in incorrect operation. If such use is necessary, this and the other devices should be
observed to ensure that they are operating correctly.
8.2 Table 1 – Compliance level for immunity tests
Guidelines and manufacturer’s declaration– electromagnetic immunity
The product is intended for use in the electromagnetic environment specified below. The user
of the product should make sure that it is used in such an environment.
Electromagnetic compatibility
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 15
Interference im-
munity tests
EN/IEC60601 test
level
Compliance level Electromagnetic envi-
ronment – guidelines
Electrostatic dis-
charge (ESD) acc.
to IEC61000-4-2
±8kV contact dis-
charge
±15kV air discharge
±8kV contact dis-
charge
±15kV air discharge
Floors should be made
of wood, concrete, or
covered with ceramic
tiles. If floors are cov-
ered with synthetic ma-
terial, the relative hu-
midity must be at least
30%.
Electrical fast
transients/bursts
acc. to IEC
61000-4-4
±2kV for power lines
±1kV for input and
output lines
100kHz repetition
±2kV for power lines
±1kV for input and
output lines
100kHz repetition
The power supply qual-
ity should be that of a
typical commercial or
hospital environment.
Surges acc. to
IEC 61000-4-5
± 1kV voltage outer
conductor – outer con-
ductor
± 2kV voltage outer
conductor – ground
± 1kV voltage outer
conductor – outer con-
ductor
± 2kV voltage outer
conductor – ground
The power supply qual-
ity should be that of a
typical commercial or
hospital environment.
Voltage dips,
short interrup-
tions, and voltage
variations acc. to
IEC61000-4-11
Voltage dip:
Dip to 0% for 1 cycle
at 0° phase angle
Dip to 70% for 25/30
cycles at 0° phase an-
gle
Dropout to 0% for 0.5
cycles @ 0°, 45°, 90°,
135°, 180°, 225°, 270°,
and 315° phase angles
Voltage interruption:
100% for 250/300cy-
cles
Voltage dip:
Dip to 0% for 1 cycle
at 0° phase angle
Dip to 70% for 25/30
cycles at 0° phase an-
gle
Dropout to 0% for 0.5
cycles @ 0°, 45°, 90°,
135°, 180°, 225°, 270°,
and 315° phase angles
Voltage interruption:
100% for 250/300cy-
cles
The power supply qual-
ity should be that of a
typical commercial or
hospital environment. If
the user of the product
requires continued op-
eration in the event of
interruptions to the
power supply network,
it is recommended that
the product be oper-
ated with an uninter-
ruptible power supply
or a battery.
Magnetic field at
the power fre-
quency (50/60Hz)
acc. to IEC
61000-4-8
30A/m at 50Hz/
60Hz
30A/m at 50Hz/
60Hz
If image distortion oc-
curs, it may be neces-
sary to install the prod-
uct further from sources
of electromagnetic
fields or to install mag-
netic shielding. Before
the product is installed,
the electromagnetic
field should be mea-
sured to ensure that it
is sufficiently low.
Immunity test acc.
to IEC61000-4–3
for radiated, ra-
3V/m 80MHz to
2.7GHz
* Refer to Table 2 for
wireless proximity RF
field test levels
3V/m 80MHz to
2.7GHz

Electromagnetic compatibility
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 16
Interference im-
munity tests
EN/IEC60601 test
level
Compliance level Electromagnetic envi-
ronment – guidelines
dio-frequency
electromagnetic
fields
Immunity to con-
ducted distur-
bances, induced
by radio-fre-
quency fields acc.
to IEC 61000-4-6
3Vrms on 150kHz to
80MHz
1kHz 80% AM modu-
lation
6Vrms in ISM band
3Vrms on 150kHz to
80MHz
1kHz 80% AM modu-
lation
6Vrms in ISM band
8.3 Table 2 – Test levels for proximity fields from HF
wireless communications equipment
Test fre-
quency
MHz
Frequency
band
MHz
Radio service Modulation Immunity
test level
V/m
Compliance
level
V/m
385 380 – 390 TETRA 400 Pulse modula-
tion 18Hz
27 27
450 430 – 470 GMRS 460,
FRS 460
FM ±5kHz
deviation
1kHz sine
wave
28 28
710 704 – 787 LTE band 13 &
17
Pulse modula-
tion
217Hz
9 9
745
780
810 800 – 960 GSM 800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE band 5
Pulse modula-
tion
18Hz
28 28
870
930
1,720 1,700 – 1,990 GSM 1800,
CDMA 1900,
GSM 1900,
DECT,
LTE band 1, 3,
4, 25,
UMTS
Pulse modula-
tion
217Hz
28 28
1,845
1,970
2,450 2,400 – 2,570 Bluetooth,
WLAN 802.11
b/g/n,
RFID 2450,
LTE band 7
Pulse modula-
tion
217Hz
28 28
5,240 5,100 – 5,800 WLAN 802.11
a/n
Pulse modula-
tion
217Hz
9 9
5,500
5,785
Electromagnetic compatibility
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 17
8.4 Table 3 – Test levels for radiated and conducted
immunity tests
Guidelines and manufacturer’s declaration– electromagnetic immunity
The product is intended for use in the electromagnetic environment specified below. The user
of the product should make sure that it is used in such an environment.
Interference immunity
tests
EN/IEC60601 test
level
Compliance
level
Electromagnetic envi-
ronment – guidelines
Conducted HF distur-
bances acc. to IEC
61000-4-6
3Vrms
150kHz to 80MHz
3Vrms Portable and mobile HF
communications equip-
ment should be used no
closer to any part of the
product, including cables,
than the recommended
separation distance calcu-
lated from the equation
applicable to the fre-
quency of the transmitter.
Recommended separation
distances:
d = 1.2√P
Where P is the rated
power of the transmitter in
watts [W] according to the
information provided by
the transmitter manufac-
turer and d is the recom-
mended separation dis-
tance in meters [m].
Field strengths from fixed
HF transmitters as deter-
mined by an electromag-
netic site survey a should
be less than the compli-
ance level in each fre-
quency range b.
d = 1.2√P
80MHz to 800MHz
d = 2.3√P
800MHz to 2.5GHz
Interferences may occur in
the vicinity of equipment
marked with the following
symbol:
Radiated HF distur-
bances acc. to IEC
61000-4-3
3V/m
80MHz to 2.5GHz
3V/m

Electromagnetic compatibility
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 18
Interference immunity
tests
EN/IEC60601 test
level
Compliance
level
Electromagnetic envi-
ronment – guidelines
Note: At 80MHz and 800MHz, the higher frequency range applies.
Note: These guidelines may not apply in all situations. The propagation of electromagnetic
waves is affected by absorptions and reflections of buildings, objects, and people.
a Field strengths from fixed transmitters, e.g., base stations for radio (cellular/cordless) tele-
phones and land mobile radios, amateur radio, AM and FM radio broadcast, and TV broad-
cast cannot be predicted theoretically with accuracy. To assess the electromagnetic environ-
ment due to fixed transmitters, an electromagnetic site survey should be considered. If the
measured field strength at the location where the device is used exceeds the above compli-
ance levels, the device should be monitored to ensure proper function. If abnormal perfor-
mance is observed, additional measures may be necessary, such as re-orienting or relocating
the product.
b Over the frequency range from 150kHz to 80MHz, field strengths should be less than 3V/
m.
8.5 Table 4 – Emission class and group
Guidelines and manufacturer’s declaration – Electromagnetic emissions
The product is intended for use in such an environment as specified below. The customer or
user of the product should ensure that it is used in such an environment.
Emission measurements Compliance Electromagnetic environment –
Guidelines
RF emissions as per CISPR 11 Group 1 The product uses RF energy only for
its internal function. Therefore, its RF
emissions are very low and are not
likely to cause any interference in
nearby electronic equipment.
RF emissions as per CISPR 11 ClassB The product is suitable for use in all
establishments including domestic
establishments and those directly
connected to the public low voltage
power supply network that supplies
buildings used for domestic pur-
poses.
Harmonic emissions as per IEC
61000-3-2
N/A
Voltage fluctuations/flicker emis-
sions as per IEC 61000-3-3
complies
8.6 Table 5 – Recommended separation distances between
portable and mobile HF communications equipment
and the product
The product is intended for use in an electromagnetic environment in which HF disturbances
are controlled. The customer or user of the product can help prevent electromagnetic
interference by maintaining a minimum distance between portable and mobile HF
communications equipment (transmitters) and the product as recommended below, according
to the output energy of the communications equipment.

Electromagnetic compatibility
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 19
Rated power of the
transmitter [W]
Separation distance d [m] according to the transmitter frequency
150kHz to 80MHz
d = 1.2√P
80MHz to 800MHz
d = 1.2√P
800MHz to 2.5GHz
d = 2.3√P
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters whose maximum rated power is not listed in the table above, the recom-
mended separation distance d in meters (m) can be estimated using the equation from the re-
spective column, whereby P is the maximum rated power of the transmitter in watts (W) ac-
cording to the transmitter manufacturer.
Note: At 80MHz and 800MHz, the separation distance for the higher frequency range ap-
plies.
Note: These guidelines may not apply in all situations. The propagation of electromagnetic
waves is affected by absorptions and reflections of buildings, objects, and people.

Subsidiaries
Instructions for use • E-BOX • OYD476_EN_V1.0_03-2021_IFU_CE-MDR 20
9 Subsidiaries
KARL STORZ SE & Co. KG
Dr.-Karl-Storz-Straße 34, 78532 Tuttlingen/Germany
Postfach 230, 78503 Tuttlingen/Germany
Phone: +49 7461 708-0 , Fax: +49 7461 708-105
Email: [email protected]
KARL STORZ Endoskope Berlin GmbH
Scharnhorststr. 3, 10115 Berlin/Germany
Phone: +49 30 3069090, Fax: +49 30 30 19452
KARL STORZ Endoscopy Canada Ltd.
7171 Millcreek Drive, Mississauga, Ontario L5N 3R3 Canada
Phone: +1 905 816-4500, Fax: +1 905 816-4599
Toll free (Canada only) Phone: 1-800-268-4880, Fax: 1-800-482-4198
(Canada only)
Email: [email protected]
KARL STORZ Endoscopy-America, Inc.
2151 East Grand Avenue, El Segundo, CA 90245-5017, USA
Phone: +1 424 218-8100, Fax: +1 424 218-8525
Toll free (USA only) Phone: 800 421-0837, Fax: 800 321-1304 (USA only)
Email: [email protected]
KARL STORZ Veterinary Endoscopy-America, Inc.
1 South Los Carneros Road, Goleta, CA 93117, USA
Phone: +1 805 968-7776, Fax: +1 805 685-2588
Email: [email protected]
KARL STORZ Endoscopia Latino-America, Inc.
815 N. W. 57th Avenue, Suite 480, Miami, FL 33126-2042, USA
Phone: +1 305 262-8980, Fax: +1 305 262-8986
Email: [email protected]
KARL STORZ Endoscopia México S.A. de C.V.
Edificio Atlantic, Oficina 3G, Calle D e/ 1ra y 3ra, 10400 Vedado, Havanna,
Cuba
Phone: +537 836 95 06, Fax: +537 836 97 76
Email: [email protected]
KARL STORZ Endoscopia México S.A. de C.V.
Av. Ejercito Nacional No. 453 Piso 2, Colonia Granada, Alcaldia Miguel
Hidalgo, C.P. 11520 Ciudad de México
Phone: +52 (55) 1101 1520
Email: [email protected]
KARL STORZ Marketing América Do Sul Ltda.
Rua Joaquim Floriano, nº. 413, 20º andar – Itaim Bibi, CEP-04534-011 São
Paulo, Brasil
Phone: +55 11 3526-4600, Fax: +55 11 3526-4680
Email: [email protected]
KARL STORZ Endoscopia Argentina S.A.
Zufriategui 627 6° Piso, B1638 CAA - Vicente Lopez, Provincia de Buenos
Aires, Argentina
Phone: +54 11 4718 0919, Fax: +54 11 4718 2773
Email: [email protected]
KARL STORZ Endoskopi Norge AS
Stamveien1, 1483 Hagan, Norway
Phone: +47 6380 5600, Fax: +47 6380 5601
Email: [email protected]
KARL STORZ Endoskop Sverige AB
Storsätragränd 14, 127 39 Skärholmen, Sweden
Phone: +46 8 505 648 00
Email: [email protected]
KARL STORZ Endoscopy Suomi OY
Taivaltie 5, 01610 Vantaa, Finland
Phone: +358 (0)96824774, Fax: +358 (0)968247755
Email: [email protected]
KARL STORZ SE & Co. KG
Representation Office
Kęstučio st. 59 / Lenktoji st. 27, 08124 Vilnius, Lithuania
Phone: +370 5 272 0448, Mobile: +370 685 67 000
Email: [email protected]
KARL STORZ Endoskopi Danmark A/S
Skovlytoften 33, 2840 Holte, Denmark
Phone: +45 45162600, Fax: +45 45162609
Email: [email protected]
KARL STORZ Endoscopy (UK) Ltd.
415 Perth Avenue, Slough, Berkshire, SL1 4TQ, United Kingdom
Phone: +44 1753 503500, Fax: +44 1753 578124
Email: [email protected]
KARL STORZ Endoscopie Nederland B. V.
Displayweg 2, 3821 BT Amersfoort, Netherlands
Phone: +31 (0)33 4545890
Email: [email protected]
KARL STORZ Endoscopy Belgium N. V.
Phone: +31 (0)33 4545890
Email: [email protected]
KARL STORZ Endoscopie France S. A. S.
12, rue Georges Guynemer, Quartier de l’Europe, 78280 Guyancourt, France
Phone: +33 1 30484200, Fax: +33 1 30484201
Email: [email protected]
KARL STORZ Endoskop Austria GmbH
Landstraßer Hauptstr. 148/1/G1, 1030 Wien, Austria
Phone: +43 1 71 56 0470, Fax: +43 1 71 56 0479
Email: [email protected]
KARL STORZ Endoscopia Ibérica S. A.
Parque Empresarial San Fernando, Edificio Munich – Planta Baja, 28830
Madrid, Spain
Phone: +34 91 6771051, Fax: +34 91 6772981
Email: [email protected]
KARL STORZ Endoscopia Italia S. r. l.
Via dell’Artigianato, 3, 37135 Verona, Italy
Phone: +39 045 8222000, Fax: +39 045 8222001
Email: [email protected]
KARL STORZ Croatia d.o.o.
Capraška 6, 10000 Zagreb, Croatia
Phone: +385 1 6406 070, Fax: +385 1 6406 077
Email: [email protected]
KARL STORZ Endoskopija d.o.o.
Cesta v Gorice 34b, 1000 Ljubljana, Slovenia
Phone: +386 1 620 5880, Fax: + 386 1 620 5882
Email: [email protected]
KARL STORZ Polska Sp. z o.o.
ul. Bojkowska 47, 44-100 Gliwice, Poland
Phone: +48 32 706 13 00, Fax: +48 32 706 13 07
Email: [email protected]
KARL STORZ Endoszkóp Magyarország Kft.
Toberek utca 2. fsz. 17/b, HU-1112 Budapest, Hungary
Phone: +36 195 096 31, Fax: +36 195 096 31
Email: [email protected]
KARL STORZ Endoscopia Romania srl
Str. Prof. Dr. Anton Colorian, nr. 74, Sector 4, 041393 Bukarest, Romania
Phone: +40 (0)31 4250800, Fax: +40 (0)31 4250801
Email: [email protected]
KARL STORZ Endoskope Greece M.E.P.E.*
Patriarhou Grigoriou E’ 34, 54248 Thessaloniki, Greece
Phone: +30 2310 304868, Fax: +30 2310 304862
Email: [email protected]
*Repair & Service Subsidiary
This manual suits for next models
1
Table of contents
Popular Control Unit manuals by other brands

Rockwell Automation
Rockwell Automation Allen-Bradley ControlLogix 1756-IB16ISOE user manual

Joy-it
Joy-it DPS BT manual

Infineon
Infineon CCU6 Interrupt 1 manual

BLAUBERG
BLAUBERG CIVIC FMM 1000 installation instructions

Wecon
Wecon LX3V-2PT2DAI-BD user manual

Xilica Audio Design
Xilica Audio Design NeuPanel Mini K1 user manual

Miele
Miele A 300/2 operating instructions

A.R.I.
A.R.I. K-010 Installation, Operating, Maintenance

System Sensor
System Sensor IM-10 Installation and maintenance instructions

Emerson
Emerson Fisher Baumann 87000 instruction manual

Panasonic
Panasonic S-LINK V instruction manual

YASKAWA
YASKAWA SI-EP3/V Technical manual