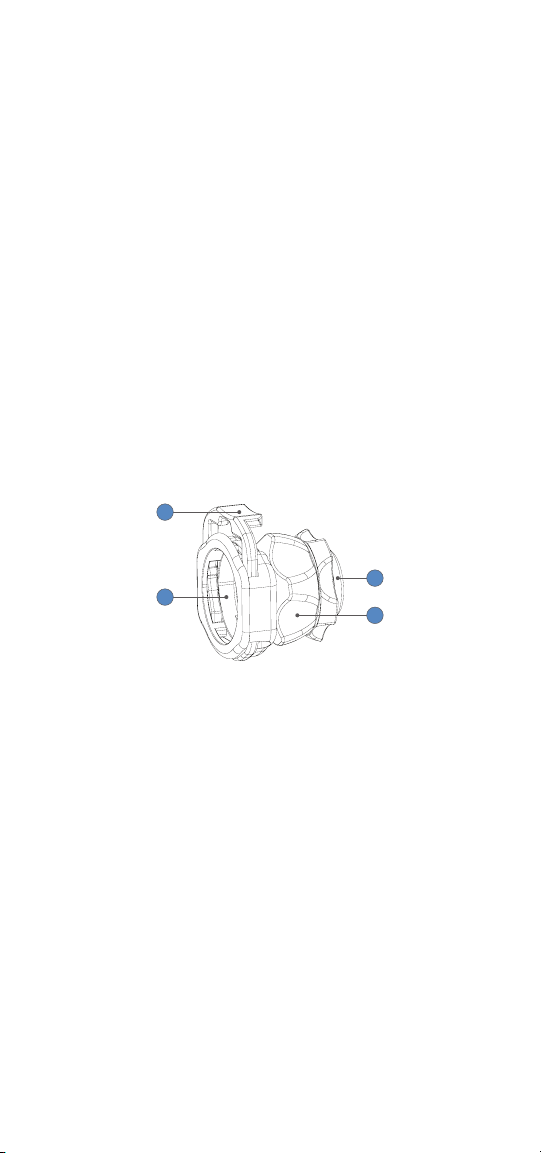
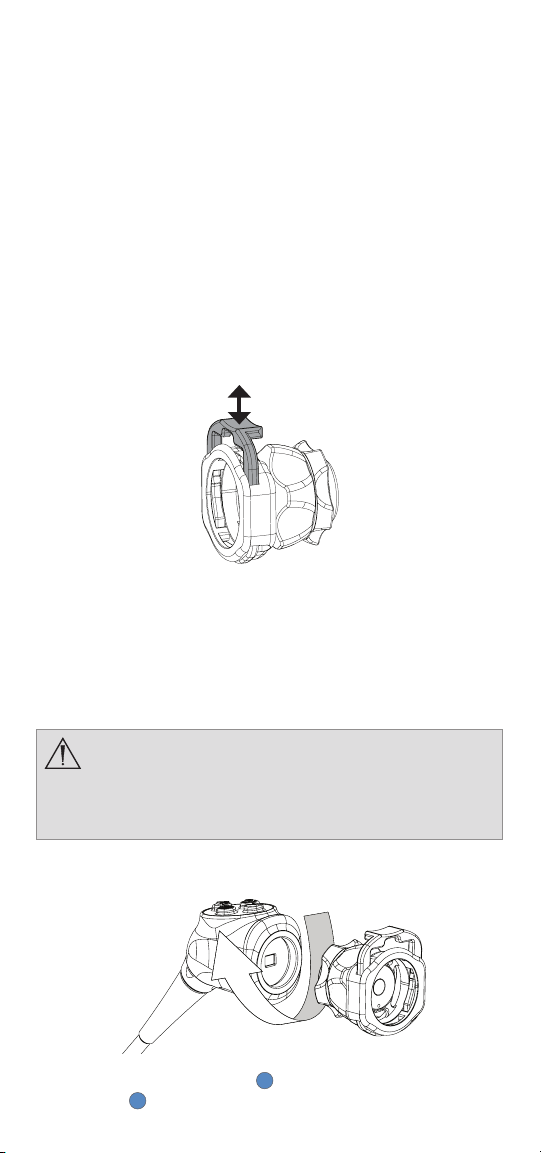
EN-4
• The sterilization parameters presented in this document apply only
when the device is sterilized outside of a sterilization tray. When
using a sterilization tray, consult the instructions provided with
the tray for proper sterilization parameters. Stryker recommends
sterilizing the device inside of a sterilization tray.
• To avoid health risks from aerosol contamination, brush the device
only when it is submerged in liquid.
• Wear appropriate protective equipment: gloves, eye protection, etc.
• Devices repaired by or purchased from third-party service
organizations could expose patients to signicant risk. These devices
are no longer validated by Stryker for cleanliness, disinfection, and
sterilization, or for safety and ecacy.
• The user shall defer to the facility’s procedures regarding
occupational exposure to bloodborne pathogens.
Cautions
• Do not use brushes or pads with metal or abrasive tips during
manual cleaning, as permanent scoring or damage could result.
• The device cannot withstand automated disinfection.
• The 4K Coupler is not autoclavable. Steam sterilizing couplers that
are not marked autoclave will result in product damage.
• To minimize galvanic corrosion, avoid soaking dissimilar metals in
close proximity.
Limitations on Reprocessing
• Do not cross-sterilize the device. Using multiple sterilization
methods can signicantly reduce the performance of the device.
• Repeated automated cleaning can degrade the product’s cosmetic
appearance.
• Damage incurred by improper processing will not be covered by the
warranty.
Materials and Equipment
All materials and equipment required to reprocess the coupler shall be
supplied by the user unless otherwise noted.
Item Description
All phases
Gloves, eye protection, etc. Wear protective equipment as
required by the medical facility
and procedure
Cleaning
Water basin Large enough to accommodate
the device
Lukewarm water To prepare cleaning solutions
Detergent1Used in cleaning solution to
remove surgical debris
Soft-bristle brush2To clean exterior or hard-to-
reach areas of the device
Reverse osmosis/
deionized water3
To rinse the device