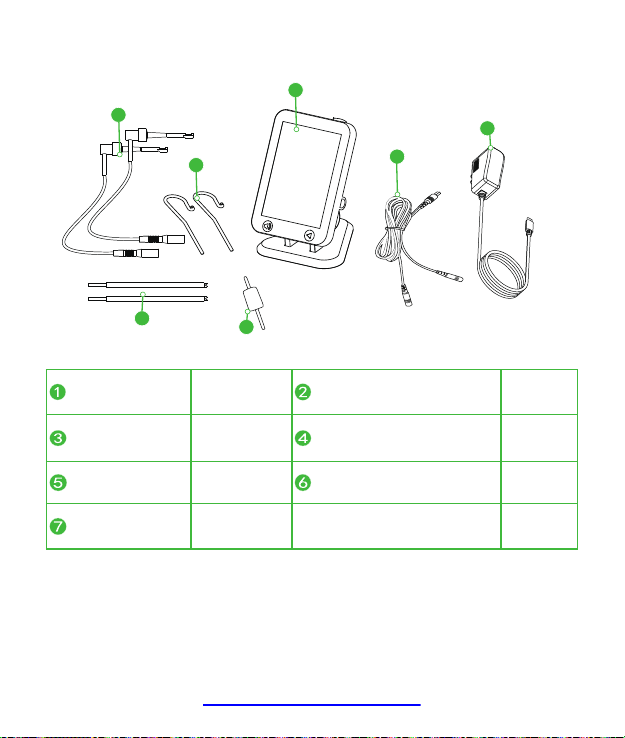
5
5. Contraindications
This equipment is contraindicated in cases where patient/user carry
medical implants such as pace makers or cochlear implants etc.
Do not use the device for implants or other non-endodontic dental
procedures.
Safety and effectiveness have not been established in pregnant
women and children.
Warring
Read the following warnings before use:
1. The device must not be placed in humid surroundings or any-
where where it can come into contact with any type of liquids.
2. The device is intended for endodontic treatment and may only
be used by trained and qualified professionals such as dentists in the
hospital.
3. Do not expose the device to direct or indirect heat sources. The
device must be operated and stored in a safe environment.
4. The device requires special precautions with regard to electro-
magnetic compatibility (EMC) and must be installed and operated in
strict compliance with the EMC information. In particular, do not use
the device in the vicinity of fluorescent lamps, radio transmitters, re-
mote controls, portable or mobile RF communication devices, and do
not charge, operate or store at high temperatures. Comply with the
specified operating and storage conditions.
5. Gloves and a rubber dam are compulsory during treatment.
6.If irregularities should occur in the device during treatment, switch
it off. Contact the agency.
7.Do not open or repair the device by yourself,otherwise, void the
warranty.