I.【SystemOperation】
1.Turningonamaindevice
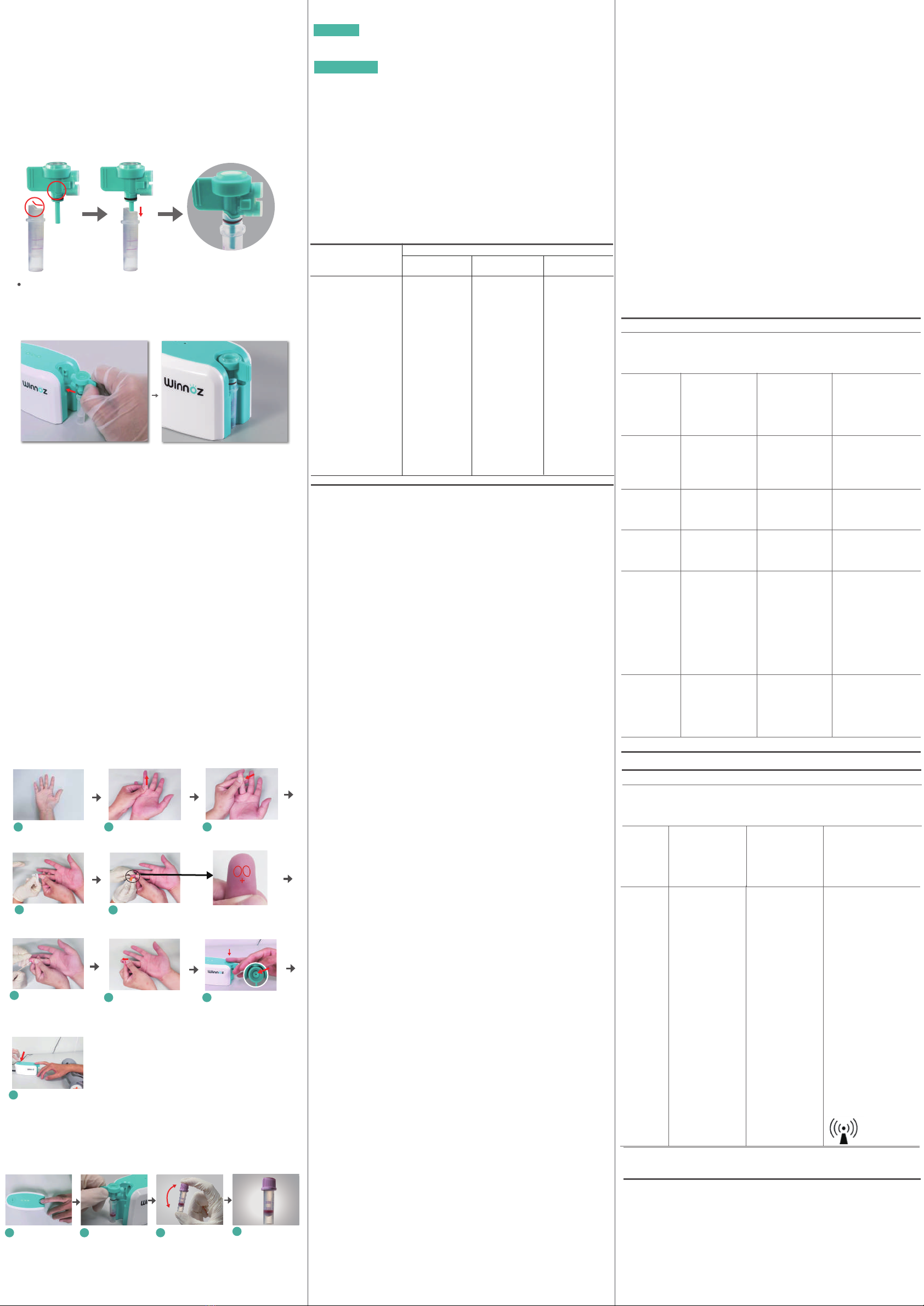
2.1Connectingacassettetoacommercialmicro-collectiontuberecommended
inthisusermanual.Pleaserefertothepicturesbelow:
2.Assemblingsteps
2.2Connectingthecassettetothemaindevice.Pleaserefertothepicturebelow:
M.【MANUFACTURERʼSdeclaration‒Electro-
magneticCompatibility‒forallMEEQUIP-
MENTandMESYSTEMS】
Manufacturerʼsdeclaration-electromagneticimmunity
TheWH-001isintendedforuseintheelectromagneticenvironment(forprofe-
ssionalhealthcare)specifiedbelow.
ThecustomerortheuseroftheWH-001shouldassurethatitisusedinsuch
anenvironment.
Immunity
Test IEC60601
TestLevel
Compliance
Level
Electromagnetic
environment-
guidance
(forprofessional
healthcare
environment)
Electrostatic
discharge(ESD)
IEC61000-4-2
Floorsshouldbewood,
concreteorceramictile.
Iffloorsarecoveredwith
syntheticmaterial,the
relativehumidityshould
beatleast30%
Contact:±8kV
Air±2kV,±4kV,
±8kV,±15kV
Contact:±8kV
Air±2kV,±4kV,
±8kV,±15kV
Electricalfast
transient/burst
IEC61000-4-4
Mainspowerquality
shouldbethatofatypical
professionalhealthcare
environment.
±2kVforpower
supplylines
±1kVforinput/
outputlines
±2kVforpower
supplylines
Notapplicable
Surge
IEC61000-4-5
±0.5kV,±1kV
line(s)toline(s)
±0.5kV,±1kV,±2kV
line(s)toearth
±0.5kV,±1kV
line(s)toline(s)
Notapplicable
Mainspowerquality
shouldbethatofatypical
professionalhealthcare
environment.
VoltageDips,
shortinterrup-
tionsandvol-
tagevariations
onpowersupp-
lyinputlines
IEC61000-4-11
Voltagedips:
0% ;0.5cycle
0% ;1cycle
70% ;25/30cycles
Voltageinterruptions:
0% ;250/300cycle
Voltagedips:
0%;0.5cycle
0%;1cycle
70%;25/30cycles
Voltageinterruptions:
0% ;250/300cycle
Mainspowerquality
shouldbethatofatypical
professionalhealthcare
environment.
IftheuseroftheWH-001
requirescontinuedopera-
tionduringpowermains
interruptions,itisrecom-
mendedthattheWH-001
bepoweredfromanunin-
terruptiblepowersupply
orabattery.
Powerfrequency
(50,60Hz)
magneticfield
IEC61000-4-8
30A/m
50Hzor60Hz
30A/m
50Hz
TheWH-001powerfre-
quencymagneticfields
shouldbeatlevelscha-
racteristicofatypicallo-
cationinatypicalprofe-
ssionalhealthcareenvi-
ronment.
NOTEisthea.c.mainsvoltagepriortoapplicationofthetestlevel.
Parallelly
Pushitinparallelly
K.【AdverseEventNotification】
Intheeventthatthereisanunexpectedaccidentordefectiveproduct,please
notifytheEuropeanauthorizedrepresentativeofWinnozTechnology,Inc.:
MedNetEC-REPGmbH:Tel:+49(0)25132266-64;Fax:+49(0)25132266-22;
Germany.
L.【Troubleshooting】
1.Causeofissue:thereisnoairtightattheinterfacesstatedbelow
2.Troubleshooting:
(a)Pleaseconfirmthatthemicro-collectiontubeiscorrectlyalignedwiththe
cassette,andtheO-ringofthecassetteshouldnotbedeformed.
(b)Pleasecheckifthecassetteissecuredaccuratelyandfirmlytothemain
device.Itisrecommendedtore-insertthecassetteintothemaindeviceand
pushthecassetteinwardtothemaindeviceslightly.
(c)PleaseadjustthepositionofyourfingertiptomakesuretheBlood
CollectionEntryiscorrectlycovered.IfyourfingertipcannotcovertheBlood
CollectionEntry,itissuggestedtotryotherfingers.
(d)Awetfingerwillaffecttheairtightofthesystem.Pleasedryyourfinger
beforethebloodcollectionprocessifyouwashedyourhandsorusean
alcoholpadtodisinfectyourfingertip.
(e)Pleasemakesurethatyourfingertipdoesnotapplypressureonthe
cassette.Itisrecommendedtorestyourarmandwristonadeskorasupport
object.
(f)Iftheissuecannotbesolvedaftertheaboveprocedures,pleasecontact
thecustomerserviceprovidedbyWinnozTechnology,Inc.
Scenario1
TheIndicatorLightsdonotturnfromyellowtobluein3-10secondsafter
pressingtheStart-Button.
Inordertowelloperatethisproduct,pleasereadthefollowinginstructionsand
correspondingpicturescarefully:
1.1Connectingamaindeviceandapoweradapter(GEM12I12-P1J)through
thePowerAdapterConnector.
1.2Connectingthepoweradaptertoapoweroutlethavingasuitablerange
ofInputRatingstatedinSectionE.
1.3MovingtheSwitchtotheONposition.
Toensureairtightduringthebloodcollectionprocess,pleaseconfirmthat
theangleofthemicro-collectiontubeopenendiscorrectlyalignedwith
thespecificdesignangleoftheTubeConnectorofthecassette.Pleasenote
thattheO-ringshouldnotbedeformedwhenassembling.
(1) Please insert the cassette into the main device and do not tilt the
cassettewheninserting.
(2) To ensure the cassette is installed firmly, please push the cassette
inwardtothemaindevice.
(1)Washanddryyourhands.
(2)SelectafingertobeprickedandputontheBloodCollectionEntry(SectionG,
2.1).Pleasekeeprelaxingyourfingertoavoidunnecessarypressureenforcedon
thecassetteandrestingyourwristandarmonadeskorasupportableobject.
Please note that the user does not need to prick the finger at this step. For
choosingafingertobepricked,pleaserefertostep4ofthisSection.
(3)PresstheStart-button,andyouwouldhearmachinerysound,feelsuctionat
your fingertip, and observe that the Indicator Lights changes from green to
yellowandthentobluein3-10seconds,indicatingthesystemhassuccessfully
enteredintothebloodcollectionmode.Ifsucceed,pleasepresstheStart-button
againfor1secondtostoptheprocessandproceedstep4tostep6ofthisSection
for the blood collection. Otherwise, please refer to Scenario 1 of Section L for
troubleshooting.
3.Checkingairtightofthesystem
Beforeconductingthebloodcollectionprocess,theusershallchecktheairtight
ofthesystembyinspectionifthemicro-collectiontubealongwiththecassetteis
correctlyinstalledonthemaindeviceperthefollowinginstructions:
5.Bloodcollectionprocedure
J.【CleaningandDisinfecting】
1.Althoughtheuserneedstooperatethemaindevicewithcleandisposable
gloves,itisstillrecommendedtocleananddisinfectthemaindevice.
2.Pleaseconfirmthatthemaindeviceisnotconnectedtoanypowersource
beforecleaninganddisinfectingit.
3.Pleasecleananddisinfectthemaindevicebeforeandaftereachuserʼsuse,
andoncethereisanydirtorbloodonthemaindevice.
4.Pleaseuseoneofthefollowingcleaningmaterials:
(a)Acleangauze,cottonswab,oranyfunctionalanalogue,moistened
withacleaningagent,includingbutnotlimitedto,CaviCide,
MEDASEPT®100oranysimilarCE-markedmedicaldevice(without
hypochlorousacidanditsderivatives)applyingtodisinfection.
(b)Anavailablecommercialdisinfectionproduct,includingbutnot
limitedto,CaviWipes,SuperSani-ClothPlusoranysimilarCE-marked
medicaldevice(withouthypochlorousacidanditsderivatives).
(c)Activityspectrumoftherecommendedcleaningagents:
5.Cleaninganddisinfectingprocedure:
(a)Wearcleandisposablegloves.
(b)Cleantheexteriorsurfacewithacleaningmaterialaforementioned.
(c)CleantheCassetteConnectorusingacottonswabmoistenedwitha
cleaningagentaforementioned.
(d)Ifthecleaningmaterialisstained,repeatabovestep5(b)or5(c)with
anewcleaningmaterial.
(e)Standandair-drythecleanedarea.
6.CAUTIONSofthecleaninganddisinfectingprocedure
(a)Beforeusinganycleaningmaterialsaforementioned,pleasecarefully
readtheIFUofthecleaningmaterialtobeused.
(b)AVOIDgettinganymoistureordirtintoanyopeningsofthemain
device.
(c)DONOTsprayanycleaningagentdirectlyontothemaindevice.
(d)DONOTuseanyorganicsolventstocleanthemaindevice.
6.Aftercompletingbloodcollection
Removethe
fingertipfrom
thecassette
Retrievethe
cassetteand
disconnectthe
micro-collection
tube
Proceedwitha
specimen
testingbased
onneeds
1234
Coverthecap.
Ifapplicable,
mixthespecimen
withthe
anticoagulant
1
Selectafingertip
tobepricked
Priority
1
2
31
2
3
> >
2
Massagethefinger
frombottomtotop
for3times
x3
Makethefingertip
congesteduntilit
ispricked
3
Disinfect
thefingertip
45
Prickthefingertip
attheSuggested
Area
Wipeoffthe
firstdropofblood
6
PresstheStart-
Buttonandavoidto
applypressureon
thecassette[Note]
9
Massagethefinger
frombottomtotop
untilablooddrop
appears
7
Gentlyplacethe
puncturesiteright
abovetheBlood
CollectionHole
8
4.Fingerselectionpriority
Therecommendedpriorityoffingersforblooddrawingarefollows:ringfinger>
middlefinger>indexfinger.Pleasenotethatthefingertipʼssizeandelasticitywill
affect the efficacy of blood collection. The fingertip's size is larger and the
fingertipismore pliable,theefficacyofblood collectionwillbeimproved.The
generalorder offingertip sizeisas shown:middle finger> index finger≈ ring
finger.Itisnotrecommendedtouseafingertiphavingcalluses.Userscanchoose
fingersdependentontheirphysicalconditions.
[Note]:Pleaserelaxyourfingerandrestyourwristandarmonadeskorasupport-
ableobject.
Cassette
Thecassetteissingle-usedisposable.Pleasediscarditasabiologicalwaste
accordingtolocalauthorityregulations.
MainDevice
-4-
1.Ifthesystemdoesnotrunproperly
Generally,onceafingertipisproperlyplacedontheBloodCollectionEntryofa
cassetteandtheStart-Buttonispressed,theIndicatorLightswillfirstturnfrom
thegreencolortotheyellowcolor,indicatingthatthemaindevicewillstartto
producenegativepressure.In3-10seconds,theIndicatorLightswillturnfromthe
yellow color to the blue color, stating that the negative pressure reaches the
preset value and the blood collection process starts. The Indicator Lights will
maintainatbluecolorfor2minutesto completethebloodcollectionprocess,
andthenthemaindevicewillgobacktothestandbymode.
IfthecolorsofIndicatorLightdonotchangeaccordingtotheabovestatements
orthemaindevicestopsduringthebloodcollectionprocess,pleaserefertothe
followingscenariosfortroubleshooting.
(a)Thecassetteandthemicro-collectiontube.
(b)TheMainDeviceConnectorofthecassetteandtheCassetteConnectorof
themaindevice.
(c)UserʼsfingertipandtheBloodCollectionEntryofthecassette.
1.Causeofissue:anotairtightstatussuddenlycausedbetweenthefingertipand
theassembledcassette.
2.Troubleshooting:
(a)PleaseadjustthepositionofyourfingertiptomakesuretheBlood
CollectionEntryiscorrectlycovered.IfyourfingertipcannotcovertheBlood
CollectionEntry,itissuggestedtotryotherfingers.
(c)Pleasemakesurethatyourfingertipdoesnotapplypressureonthe
cassette.Itisrecommendedtorestyourarmandwristonadeskorasupport
object.
(d)Iftheissuecannotbesolvedaftertheaboveprocedures,pleasecontact
thecustomerserviceprovidedbyWinnozTechnology,Inc.
(e)Ifyouneedtostoptheprocess,pleasepresstheStart-Buttonfor1second.
Scenario2
Duringthebloodcollectionprocess,theIndicatorLightsturnfromthe
bluecolortotheyellowcolor.
2.Iftheusercouldnotcollectenoughbloodvolumeorqualityblood
Duetomultipleheterogeneous factorssuchas age,microvasculardistribution,
bloodcirculation,atmosphericpressure,etc.,thebloodvolumecollectedwiththe
productcouldvaryindifferentscenarios.Notwithstanding,whensufficientblood
specimenscannotbecollected,pleasetrythefollowingmethodstoobtainmore
blood or seek specialized medical assistance or try other blood collection
methods.
(a)Beforebloodcollection,itisrecommendedthatyouwarmandmassageyour
fingersproperly.
(b)Accordingtoexperimentaldata,hyperhidrosisandhypotensionmayaffect
thebloodvolumecollectedbecauseawetfingerwillaffecttheairtightofthe
system.Pleasedryyourfingerbeforethebloodcollectionprocessifyouwashed
yourhandsoruseanalcoholpadtodisinfectyourfingertip.
(c)Ifyouhadappliedhandcreamonyourhand,pleasewashitawayanddry
yourfingersasitmaycontaminatethebloodspecimen.
(d)Theairtightofthesystemaffectsthecollectedbloodvolume.Pleaseconfirm
thesystemisairtightbeforebloodcollectionbyfollowingtheinstructionsinthe
scenario1ofSectionL.
(e) When operating this product, it is necessary to use a blood lancet with a
needle(diameter≥0.8mm,gaugenumber≤21G)orablade(width≥1.5mm).
Inaddition,usingalancetwhosediameterorwidthissmallerthanthatof21G
wouldincreasehemolysisrisk.
(f)Supposebloodcannotbesuccessfullycollectedafterfollowingthecustomer
serviceʼsguidanceandconfirmingthattheproductfunctionsnormallyandhas
beenoperatedcorrectly,theproductmaynotbeapplicabletothisuser.
-5-
IFU-1801
EN2020-09rev.5.7
-6-
TheWH-001isintendedforuseintheelectromagneticenvironment(forprofe-
ssionalhealthcare)specifiedbelow.
ThecustomerortheuseroftheWH-001shouldassurethatitisusedinsuch
anenvironment.
Immunity
Test IEC60601
TestLevel
Compliance
Level
Electromagnetic
environment-
guidance
(forprofessional
healthcare
environment)
Manufacturerʼsdeclaration-electromagneticimmunity
NOTE1At80MHzand800MHz,thehigherfrequencyrangeapplies.
NOTE2Theseguidelinesmaynotapplyinallsituations.Electromagneticpropagation
isaffectedbyabsorptionandreflectionfromstructures,objectsandpeople.
ConductedRF
IEC61000-4-6
RadiatedRF
IEC61000-4-3
3Vrms:
0.15MHz-80MHz
6Vrms:
inISMbandsbetween
0.15MHzand80MHz
80%AMat1kHz
3V/m
80MHz‒2.7GHz
80%AMat1kHz
3Vrms:
0.15MHz-80MHz
6Vrms:
inISMbandsbetween
0.15MHzand80MHz
80%AMat1kHz
3V/m
80MHz‒2.7GHz
80%AMat1kHz
PortableandmobileRF
communicationsequipment
shouldbeusednocloserto
anypartoftheWH-001
includingcables,thanthe
recommendedseparation
distancecalculatedfromthe
equationapplicabletothe
frequencyofthetransmitter.
Recommendedseparation
distance:
=1.2√
=1.2√80MHzto800MHz
=2.3√800MHzto2.7GHz
Where
isthemaximum
outputpowerratingofthe
transmitterinwatts(W)accor-
dingtothetransmittermanu-
facturerand
istherecom-
mendedseparationdistance
inmetres(m).
Interferencemayoccurinthe
vicinityofequipmentmarked
withthefollowingsymbol:
SuggestedArea
(+:thecenterof
thefingerprint)
Recommendedreactiontime
MEDASEPT®
100
SuperSani-
ClothPlus
30 sec.
30 sec.
30 sec.
30 sec.
1 min.
1 min.
N.A.
2 min.
30 sec.
5 min.
N.A.
1 min.
N.A.
N.A.
30 sec.
30 sec.
30 sec.
30 sec.
30 sec.
30 sec.
15 sec.
15 sec.
15 sec.
15 sec.
N.A.
N.A.
3 min.
3 min.
N.A.
30 sec.
N.A.
N.A.
Activity
Spectrum CaviCide/
CaviWipes
Bactericidal
Fungicidal
Virucidal
Mycobactericidal/
Tuberculocidal
Pseudomonas aeruginosa
Staphylococcus aureus
Enterococcus spp. (incl. VRE)
MRSA
3 min.
3 min.
3 min.
3 min.
Candida albicans
Aspergillus brasiliensis
Trichophyton mentagrophytes
1 min.
1 min.
3 min.
HBV/HCV/HIV
Influenza A2/H1N1/H5N1
Vaccinia virus
Coronavirus
HSV-1/HSV-2
BVDV
2 min.
2 min.
2 min.
2 min.
2 min.
2 min.
Mycobacterium terrae
Mycobacterium avium
Mycobacterium bovis
1 min.
1 min.
3 min.
Note
N.A. is for Not Available.