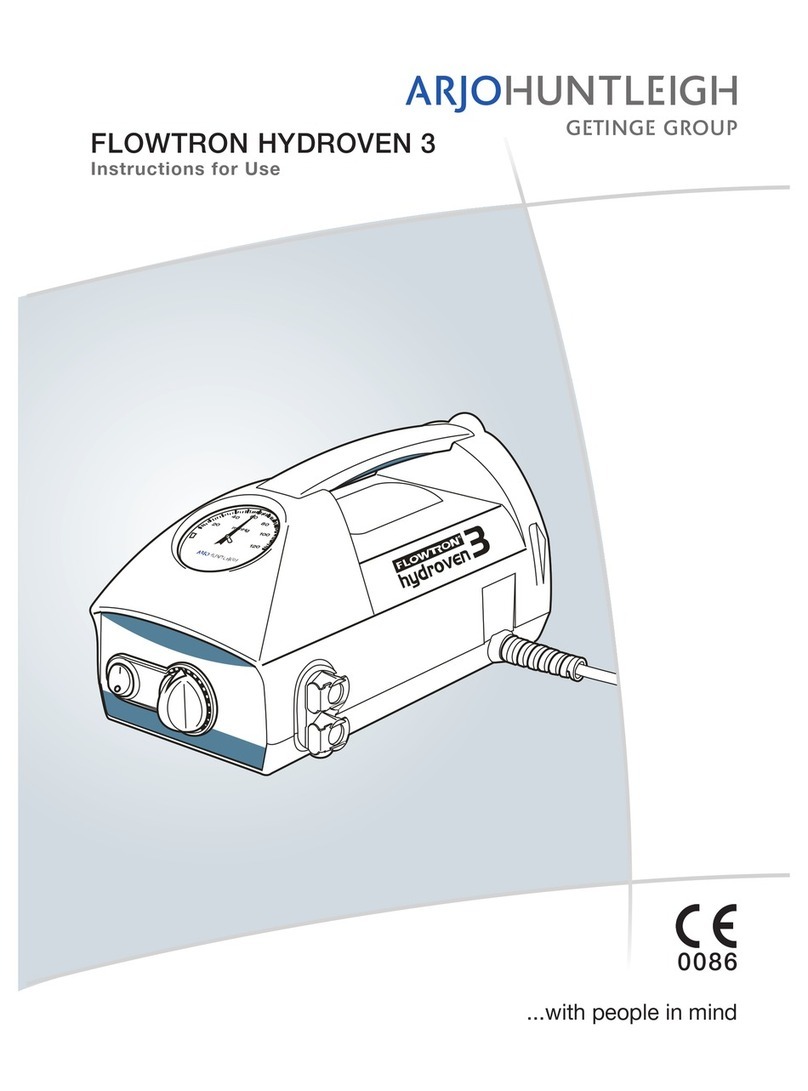
(iii)
GENERAL SAFETY
Before you connect the system pump to a mains socket, read carefully all the installation
instructions contained within this manual.
The system has been designed to comply with regulatory safety standards including:
• EN60601-1:1990/A13:1996 and IEC 60601-1:1988/A2:1995
• UL60601-1, UL2601-1 and CAN/CSA C22.2 No. 601.1-M90
• EN60601-1:2006 and IEC 60601-1:2005
• AAMI/ANSI ES60601-1:2006 and CAN/CSA C22.2 No.60601.1(2008)
Safety Warnings
• It is the responsibility of the care giver to ensure that the user can use this product
safely.
• Make sure that the mains power cable and tubeset or air hoses are positioned to
avoid causing a trip or other hazard, and are clear of moving bed mechanisms or
other possible entrapment areas.
• Electrical equipment may be hazardous if misused. There are no user-serviceable
parts inside the pump. The pump's case must only be removed by authorised
technical personnel. No modification of this equipment is allowed.
• The mains power socket/plug must be accessible at all times. To disconnect the
pump completely from the electricity supply, remove the plug from the mains
power socket.
• Disconnect the pump from the mains power socket before cleaning and inspecting.
• Keep the pump away from sources of liquids and do not immerse in water.
• Do not use the pump in the presence of uncontained flammable liquids or gasses.
• Only the pump and garment/insert combination as indicated by ArjoHuntleigh
should be used. The correct function of the product cannot be guaranteed if
incorrect pump and garment combinations are used.
Caution (applicable to the USA market only)
• US Federal law restricts this device to sale by or on the order of a physician.
Precautions
For your own safety and the safety of the equipment, always take the following precautions:
• Do not expose the system to naked flames, such as cigarettes, etc.
• Do not store the system in direct sunlight.
• Do not use phenol-based solutions to clean the system.
• Make sure the system is clean and dry prior to use or storage.
• Pets and children must be supervised in the vicinity of the system.
Electromagnetic Compatibility (EMC)
This product complies with the requirements of applicable EMC Standards. Medical electrical
equipment needs special precautions regarding EMC and needs to be installed in accordance
with the following instructions:
• The use of accessories not specified by the manufacturer may result in increased
emissions by, or decreased immunity of, the equipment, affecting its performance.
• Portable and mobile radio frequency (RF) communications equipment (e.g. mobile/cell
phones) can affect medical electrical equipment.