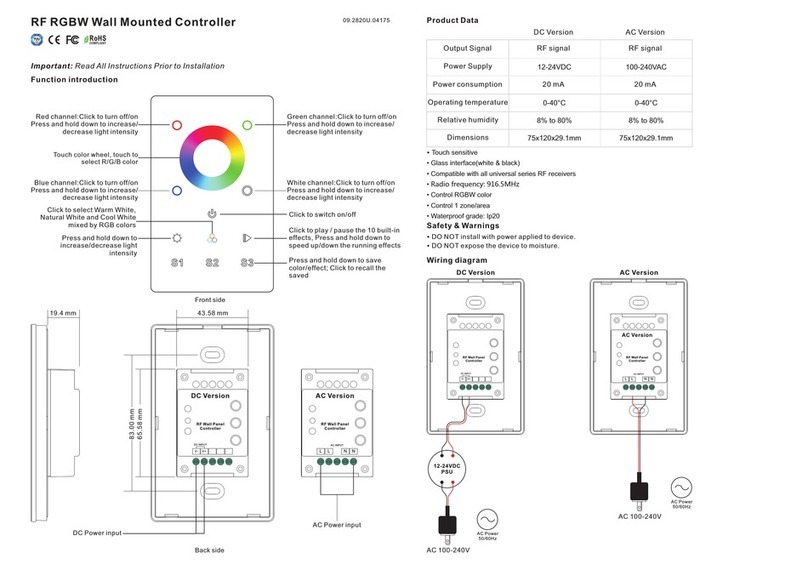
Biegler autopress e User manual

Instructions for use
automatic pressure controller
Version 2020-08-27
40-203-02
ENG
E
N
G

2
IMPORTANT
These directions are essential for operating the device. They must
therefore be kept in a suitable place near the device, and should be kept
with the device if it is given to other users.
For proper and safe use of this device, it is essential that the following
warnings and safety instructions, as well as the operating instructions,
are read and carefully observed by all users before first using the device.
It is the responsibility of those using the device to fully acquaint
themselves with its proper use and operation. If a malfunction is
suspected, the device is to be taken out of service immediately and
suitable warning signs should be attached to the device to ensure that it
is not used before the required service and repair work has been carried
out.

3
Table of Contents
1. Warnings and Safety Instructions.........................................................4
2. Description..............................................................................................6
2.1 General Description..........................................................................6
2.2 Scope of Delivery.............................................................................7
2.3 Intended use....................................................................................7
2.4 Indication..........................................................................................7
2.5 Contraindication...............................................................................7
3. Initial Operation......................................................................................8
4. Maintenance..........................................................................................10
5. Cleaning and Disinfection....................................................................10
6. Periodic Inspections.............................................................................10
7. Manufacturer Liability ..........................................................................11
8. Warranty Conditions ............................................................................12
9. Return of Devices.................................................................................13
10.Disposal ................................................................................................13
11.Symbols ................................................................................................14
12.Operating and Storage Conditions......................................................15
13.Electromagnetic Compliance Levels...................................................15
13.1 Emission ........................................................................................15
13.2 Immunity Test Levels .....................................................................15
14.Technical Data......................................................................................17
15.Manufacturer’s Declaration..................................................................18
16.Manufacturer.........................................................................................18

4
1. Warnings and Safety Instructions
xIn the event of any suspected malfunction while in operation, the device should
be immediately removed from service.
xUnplugging the mains plug is the only safe way to disconnect from the mains
power supply.
xThe device may only be fastened to infusion stands, tripods or equipment rails
which have sufficient stability and load capacity to support the device.
xOnly pressure infusion bags specified by BIEGLER or approved by BIEGLER
for use with this device may be used in conjunction with the DXWRSUHVVŹH.
xThe device must only be used in areas in which the electrical installations are in
accordance with the standards and regulations in force.
xThe device must not be used in rooms with potential explosion hazard.
xThe device must not be immersed in liquids or sterilized with steam or by
thermochemical methods.
xAll extraneous influences such as electromagnetic waves or high temperatures
are to be kept to a minimum.
xAvoid exerting force on the device or its accessories.
xIf the device is dropped, damaged due to force, or functions in a way other than
described in the operating instructions, stop using the device immediately and
return it to the service center.
xPeriodic technical safety inspections must be carried out as described in the
"Periodic inspections" section.
xPersons and services authorized by BIEGLER must carry out repairs and
modifications on the device.
xNo mechanical or electrical changes may be made to the components of the
device.
xImproper replacement of the lithium battery (other model) would result in an
unacceptable risk. The lithium battery may only be replaced by BIEGLER or by
authorized BIEGLER personnel.
xReplacement of the lithium accumulator by insufficiently trained personnel can
lead to hazards.

5
xOnly infusion bags that are capable of 300 mmHg must be used. Adhere to the
instructions for use for this bags.
xThe pressure infusion bags must be securely fastened at least 20 cm above the
patient to prevent air embolism. Prevent the connecting hose from kinking.
xAlways position the DXWRSUHVVŹH in such a way that it is easy to operate without
obstacles.No other devices or infusion stands shall be positioned shortly before.
xMake sure, that mains plug of the DXWRSUHVVŹH is easily reachable to the
operator. (The mains plug is used to disconnect the device from mains)
xThe automatic pressure controller DXWRSUHVVŹH is a class I ME equipment and
therefore only intended to be connected to supply mains with protective earth.
The DXWRSUHVVŹH may not be used if:
xthe housing is damaged or one of the front film layers becomes detached
xthe device has been exposed to a hard physical shock (e.g. dropped, hit or
shaken)
xthe device has been immersed in water
xthe mains power cord or plug is damaged
xthe device has given someone an electric shock
xthe fixing clamp is damaged in such a way that safe clamping to the infusion
stand is no longer guaranteed.
The DXWRSUHVVŹH may not be used if there is a malfunction:
e. g. display error, no pressure, …
If there is a malfunction, suitable warning signs should be attached to the device
to ensure that it is not used until the required service and repair work has been
carried out.
Safety instructions for disposable pressure infusion bags:
Disposable consumable materials are intended for single use only. The re-use of
products that are intended for single use constitutes a risk of infection for patient
or user.

6
Safety instructions for reusable pressure infusion bags:
Please refer to the instructions for use for reusable pressure infusion bags.
2. Description
2.1 General Description
Description Article number
DXWRSUHVVŹH LG4000004
The automatic pressure controller DXWRSUHVVŹH is used when liquids are to be
supplied under constant pressure.
The DXWRSUHVVŹH can be used in combination with all BIEGLER Blood- and
Infusionwarmers or used as standalone device.
The following single-use pressure infusion bags can be used:
Description Article number
Pressure Infusion Bag 500 ml JR2000500
Pressure Infusion Bag 1000 ml JR2001000
Pressure Infusion Bag 3000 ml JR2003000
The following reusable pressure cuffs may also be used:
Description Article number
BIEGLER Reusable Pressure Infusion Bag 500 ml JR5000500
BIEGLER Reusable Pressure Infusion Bag 1000 ml JR5001000
BIEGLER Reusable Pressure Infusion Bag 3000 ml JR5003000

7
2.2 Scope of Delivery
Quantity Description
1DXWRSUHVVŹH
2Pressure Infusion Bag 500 ml
1Instruction for use
2.3 Intended use
The automatic pressure controller DXWRSUHVVŹH is designed for use in IV infusion
therapy and for applications where IV infusions or irrigations are to be supplied
under constant pressure.
2.4 Indication
The automatic pressure controller DXWRSUHVVŹH can be used wherever liquids are
to be administered under pressure.
2.5 Contraindication
The automatic pressure controller DXWRSUHVVŹH does not provide significant data
to use the device as a flow measurement or control system. Therefore, the device
must not be used as a replacement for infusion pumps or to control the
administration of medication.
The automatic pressure controller DXWRSUHVVŹH must not be used for enteral
nutrition solutions.

8
3. Initial Operation
Observe the instructions for use! Handling of this device requires
knowledge and adherence to these instructions. The autopressŹe
and accessories may only be used by physicians, physician
assistants or other qualified specialized staff. The condition of the
patient has to be monitored during the application.
1.Fix the DXWRSUHVVŹH firmly on a stand using the clamps at
the back. Only use infusion stands, tripods or equipment
rails that are sufficiently stable.
2.Before connecting to the mains power supply, check that the
voltage specified on the device label matches the mains
voltage.
3.Before you turn on the device, insert the infusion bag or
fluid bag into a suitable pressure cuff. Make sure that air
has been properly removed from the infusion or flush
system before connecting it to the patient.
4.Connect the pressure infusion bag to the Luer-Connector
of the DXWRSUHVVŹH
5.Switch on the device by pressing the button in the upper
right.
6.Set the desired pressure with the arrow buttons and .
(in steps of 10 mmHg)
7.Start the pressure build-up by pressing the START-buttons or to the
corresponding channel.
8.If necessary, the pressure in both channels can be changed by pressing the
and buttons.
9.By pressing the PAUSE-button the pressure in the corresponding channel is
released and the channel is vented.
During patient transfer, the device can be disconnected from the mains without
switching off. The lithium accumulator provides the power supply for the device
and the pressure in the bag is maintained.
Figure 1 - placing the
infusion bag

9
Figure 1 - Operation, Display
battery indicator
Operation:
ON / Standby
activate right
channel
activate left
channel
decrease
pressure
increase
pressure
vent right
channel
vent left
channel
set pressure
ratio actual
to set
pressure
actual pressure
clamp
power supplied
Standby-LED
channel vented
(symbol disappears
when channel is
activated)

10
4. Maintenance
The DXWRSUHVVŹH was largely designed as a maintenance-free device. For long-
term maintenance of quality and functional safety, we would like to ask you to
observe the following points:
xAlways keep the device clean (see the "Cleaning and disinfection" section).
xPeriodic technical safety inspections must be carried out as described in the
"Periodic inspections" section.
5. Cleaning and Disinfection
Important: Before cleaning or disinfecting, the device must always be
disconnected from the mains power supply by pulling the plug.
The device may only be cleaned using a soft cloth with a water-soluble, non-
aggressive cleaning agent or a special cleaning agent for plastics.
For the purposes of disinfection, only ready-made alcohol-based disinfectants
must be used and the manufacturer ‘s instructions must be followed.
6. Periodic Inspections
The periodic technical safety inspections (according to the local standards in force
- e.g. in Austria, EN 62353) on the DXWRSUHVVŹH must be carried out at least every
12 months, by persons authorized to carry out such inspections based on their
training, knowledge and practical experience. The device cannot be used during
inspection. The pressure limitation, as essential performance of the device, is
tested during inspection.
xThe safety relevant labels on the device and its accessories must be clearly
legible.
xThe mechanical condition of all components must permit further safe use
(housing, power cable, Luer connections).
xThe device must not contain any safety-reducing contamination.

11
The results of the periodic inspection must be documented, along with the date,
the inspecting agency and the device number.
Important: If a malfunction is discovered during the periodic inspection,
suitable warning signs should be attached to the device to ensure that it
is not used before the required service and repair work has been carried
out.
7. Manufacturer Liability
The manufacturer and the supplier of the device reject any liability if:
xthe device is not used in accordance with the Instructions for use.
xthe user is not sufficiently informed about the functioning of the device
corresponding with the Instructions for use and the safety instructions
xrepairs are not performed exclusively by the manufactureror by persons and
service centers expressly authorized by the manufacturer
xthe device is used in places in which the electrical installations do not comply
with the applicable national standards, or if the power supply during the
period of use of the device is not guaranteed
xoriginal spare parts are not used or the maintenance interval is not adhered
to.

12
8. Warranty Conditions
The manufacturer guarantees that all flaws of material and workmanship which
arise within 24 months from the date of purchase will be repaired free of charge.
Claims are only accepted under the following terms:
xThe manufacturer and/or supplier is informed immediately of the fault for
which the warranty claim is being made.
xThe instructions of the manufacturer and/or supplier regarding storage or
return of the device are complied with.
xPresentation of a legible copy of the invoice for the device concerned,
showing the date of purchase.
xAn exact description of the defects or malfunctions identified by the customer.
The manufacturer's warranty will be void if it is found that the maintenance,
disinfection and inspection instructions have not been followed according to the
operating instructions, the device has been damaged by force or operating error,
or has been used in any way contrary to the operating and safety instructions. The
warranty will also be void if original BIEGLER materials were not used as
replacement parts, or measures for repair were undertaken by persons not
authorized by the manufacturer or supplier.
If the manufacturer is required to meet a warranty claim in accordance with these
terms, the customer shall bear the costs and risks of transport of the device from
and to the place of use.
The manufacturer and/or supplier shall under no circumstances assume liability
for slight negligence. The compensation for lost earnings and profits is likewise
excluded.

13
9. Return of Devices
Devices must be carefully cleaned and disinfected before being placed in the
original packaging for returning.
If the original packaging is no longer available, the product has to be suitably
packaged for the method of dispatch.
10. Disposal
Dispose of the device or its accessories in accordance with local regulations.
Do not dispose of this product
as unsorted municipal waste

14
11. Symbols
Compliance with Directive
93/42/EEC Serial number
Consult instructions for use Manufacturer
A
ttention Manufacturing date
Defibrillation-proof type CF
applied part
ON / Standby
A
C voltage
A
ctivate right channel Increase pressure
A
ctivate left channel Decrease pressure
Pause button –
vent the channel IPX4 Degree of protection
against ingress of water -
splashing water
Temperature limit Humidity limitation
Fragile, handle with care Protect from heat and
radioactive sources
Keep dry Do not dispose of this
product as unsorted
municipal waste

15
12. Operating and Storage Conditions
Permissible environmental conditions for transporting and storing the
DXWRSUHVVŹH and accessories:
Transport and storage Operating
Temperature 10 – 40 °C 10 – 30 °C
Relative humidity 30 – 75 % 30 – 75 %
Ambient pressure 700 – 1060 hPa 700 – 1060 hPa
Values higher or lower than the ranges specified above may cause
damage to the device or its accessories.
13. Electromagnetic Compliance Levels
13.1 Emission
Test Limit
Conducted emission CISPR 11, Group 1, Class B
Radiated emission CISPR 11, Group 1, Class B
Harmonic current emissions
(IEC 61000-3-2) IEC 61000-3-2, Class A
Voltage fluctuations and flicker
(IEC 61000-3-3) IEC 61000-3-3, Complies
13.2 Immunity Test Levels
Test Test level
Electrostatic Discharge
(IEC 61000-4-2) Contact Discharge: ±8 kV
Air Discharge: ±2 kV, ±4 kV, ±8 kV, ±15 kV
Radiated RF EM filed
(IEC 61000-4-3) 80-2700 MHz; 1kHz AM 80 %; 3 V/m

16
Proximity fields form RF
wireless
communications
equipment
(IEC 61000-4-3)
385 MHz;
Pulse Modulation: 18 Hz; 27 V/m
450 MHz,
Pulse Modulation: 18 Hz: 1 kHz sine; 28 V/m
710, 745, 780 MHz;
Pulse Modulation: 217 Hz; 9 V/m
810, 870, 930 MHz;
Pulse Modulation: 18 Hz; 28 V/m
1720, 1845, 1970 MHz;
Pulse Modulation: 217 Hz; 28 V/m
2450 MHz;
Pulse Modulation: 217 Hz; 28 V/m;
5240, 5500, 5785 MHz;
Pulse Modulation: 217 Hz; 9 V/m
Electrical fast transients
/bursts
(IEC 61000-4-4)
Power lines: 2 kV; 100 kHz repetition frequency
Signal lines: 1 kV; 100 kHz repetition frequency
Surges
(IEC 61000-4-5) L-PE and N-PE: 2kV
L-N: 1kV
Conducted disturbances
inducted by RF fields
(IEC 61000-4-6)
0.15-80 MHz; 1kHz AM 80 %; 3 Vrms, 6 Vrms
in ISM Band
Rated power frequency
magnetic fields
(IEC 61000-4-8) 30 A/m, 50 Hz and 60 Hz
Voltage dips / Voltage
interruptions
(IEC 61000-4-11)
0 % UTfor 0.5 cycle at 8 phase angles
0 % UTfor 1 cycle at 0°
70 % UTfor 25/30 cycles at 0°
0%U
Tfor 250/300 cycles at 0°

17
14. Technical Data
Device: automatic pressure controller
Type designation: DXWRSUHVVŹH
Operating voltage: 100 – 240 V / 50 – 60 Hz
Power consumption: max. 36 VA
Supply type: mains or battery operation
Protection class: I
Degree of protection
against electric shock: Type CF, defibrillationproof
IP-classification (IEC 60529): IPX4
Classification (93/42/EEC): IIa according to Rule 9
Operation mode: continuous
Pressure range: 0 – 300 mmHg
Accuracy of values displayed: ± 5% of the measured value
Dimensions: 100 x 230 x 180 mm
Weight (device only): 2.3 kg
Battery specification
Type: Li-ion battery
(Type designation: FB3S1P18650-26)
Charging: automatically when device is connected
to mains
Charging time: max. 6.5 hours if battery discharged
Mean time to empty battery 3 hours @ continuous pumping and
from full charge: 20°C ambient temperature
Applied part: venous access (not included)
Figure 2 – Applied part depiction
to patient

18
15. Manufacturer’s Declaration
The DXWRSUHVVŹH is a medical product as defined by Directive 93/42/EEC.
This is documented through the CE mark.
Notified Body: TÜV SÜD Product Service GmbH,
Approval Number CE0123
16. Manufacturer
Biegler GmbH
Allhangstrasse 18a
3001 Mauerbach
Austria
Tel. +43 1 979 21 05
Fax +43 1 979 21 05 16
email: office@biegler.com
www.biegler.com
This manual suits for next models
1
Table of contents
Popular Controllers manuals by other brands

Mitsubishi Electric
Mitsubishi Electric CR750-D Series instruction manual

PXM
PXM Wireless DMX manual

emaux
emaux RO 7 Manual Installation

MarelliGenerators
MarelliGenerators General Power M63FA310A user manual

Siemens
Siemens SPC3 Hardware description

Baker Hughes
Baker Hughes Mooney 21 Series instruction manual