BLACK & BLACK SURGICAL VITRUVIAN B89025 Manual
DOC # 25-3012 REV L 09/2021 Page 1of 29
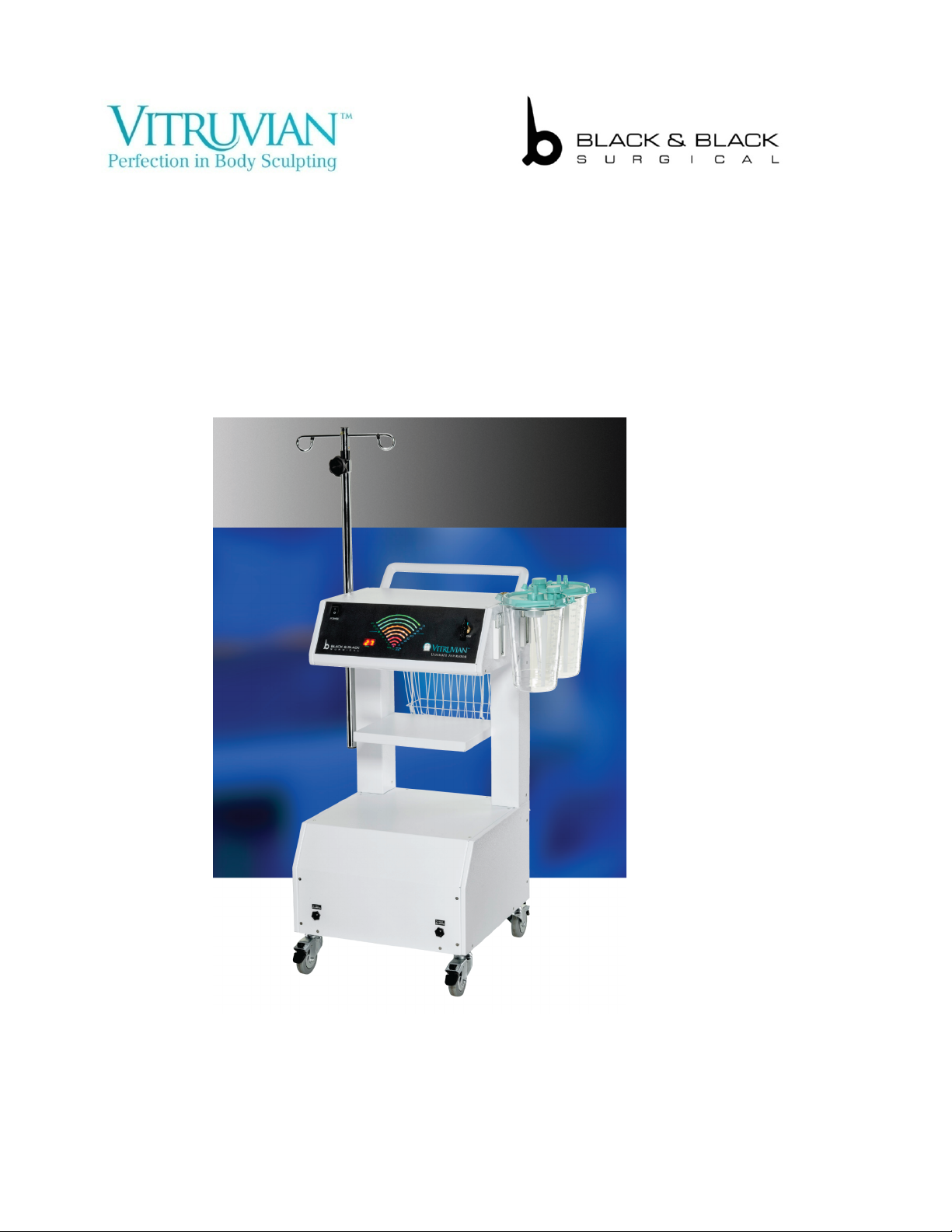
B89025 VITRUVIAN™ULTIMATE
ASPIRATOR
120V/230V
Instructions for Use Manual
DOC # 25-3012 REV L 09/2021 Page 2of 29
CONGRATULATIONS
You have purchased one of the finest aspirators available. Utilizing two piston driven
½ HP motors assures that you are able to achieve and maintain the highest vacuum
levels that the laws of physics allow. Beyond that you will have complete control over
the vacuum level giving you much more versatility in how you use the device. The
motors that were selected for this device are also some of the quietest available
utilizing a muffler on each along with noise insulated cabinet which make it one of the
quietest aspirators available. Read this entire booklet before trying to use your
aspirator.
CALL US
Our customer service department is staffed with friendly, knowledgeable people who
are ready to help. Whether you need replacement disposables or assistance in
troubleshooting an issue, they will help you or put you in touch with someone who
can.
TOLL FREE: 1-877-252-2517
PHONE: 1-770-414-4880
FAX: 1-770-414-4879
0297
MEDAGENT International
GmbH
Griesweg 47
78570 Mühlheim
Tel.: +49.7463.9954.0
Fax: +49.7463.9954.10

DOC # 25-3012 REV L 09/2021 Page 3of 29
Instructions for Use
Table of Contents
Table of Content
Table of Contents.........................................................................................................................................3
I. Introduction................................................................................................................................................5
Symbol Definitions...........................................................................................................................................5
Use of This Documentation .............................................................................................................................5
Warnings......................................................................................................................................................6
Precautions...................................................................................................................................................6
Operational Safety ...........................................................................................................................................7
Modifications ...................................................................................................................................................8
Damage or Loss in Shipment...........................................................................................................................8
Incorrect Items Shipped by Black & Black Surgical .......................................................................................8
Policy on Returned Goods ...............................................................................................................................9
Return Shipping ...............................................................................................................................................9
Repair Program .............................................................................................................................................10
Warranty Policy.............................................................................................................................................10
II. Operation..................................................................................................................................................11
Intended Use ..................................................................................................................................................11
Intended User /Patient Population / Environment ........................................................................................11
Contraindications ..........................................................................................................................................11
Unpacking......................................................................................................................................................12
Items Included with your device:...................................................................................................................12
Initial Inspection, Set Up, and Testing ..........................................................................................................13
Operative Controls and Components ............................................................................................................14
Additional Features .......................................................................................................................................18
Backup Operation of Device..........................................................................................................................18
(If using Black & Black parts) Canister Set-Up Illustration .........................................................................19
Usage Instructions .........................................................................................................................................21

DOC # 25-3012 REV L 09/2021 Page 4of 29
Suggestions ....................................................................................................................................................21
III. Maintenance ..........................................................................................................................................22
Cleaning Instructions.....................................................................................................................................22
Troubleshooting Guide ..................................................................................................................................23
IV. Warranty Provisions ............................................................................................................................24
APPENDIX – A..............................................................................................................................................25
VITRUVIAN™Ultimate Aspirator Specifications...................................................................................25
Table 1 – Guidance and manufacturer’s declaration – electromagnetic emissions .....................................26
Table 2 – Guidance and manufacturer’s declaration – electromagnetic immunity......................................27
Table 4 – Guidance and manufacturer’s declaration – electromagnetic immunity......................................28
Table 6 – Recommended separation distances between portable and mobile RF communications equipment
and the VITRUVIAN™Ultimate Aspirator ...................................................................................................29
DOC # 25-3012 REV L 09/2021 Page 5of 29
I. Introduction
Symbol Definitions
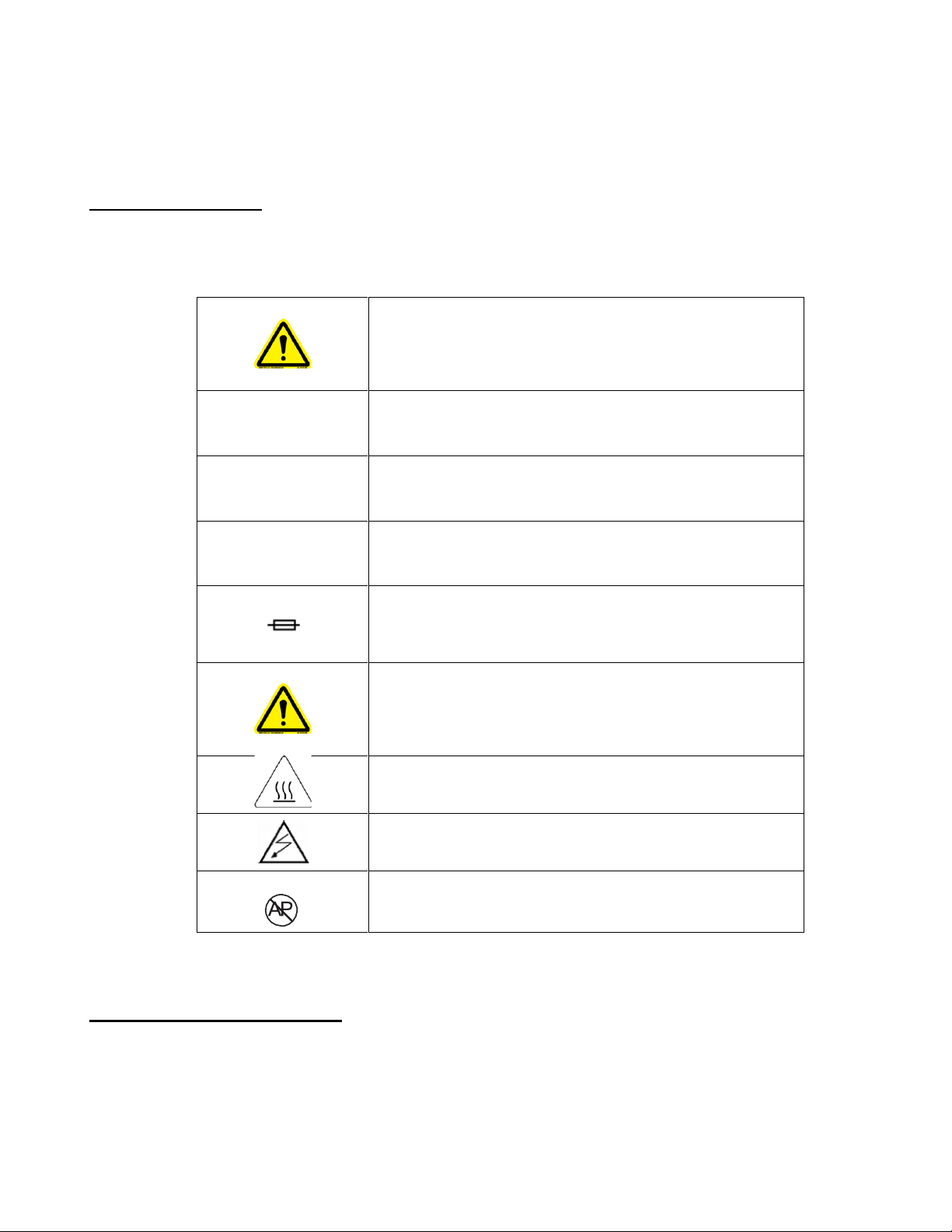
The following symbols appear on the exterior of the product or elsewhere in this User’s Manual.
Attention: Consult accompanying documents
O
Power OFF
I
Power ON
~
Alternating Current
Fuse
Attention: Consult User’s Manual
USE PROPER GROUNDING: Use only receptacle
marked “hospital Grade”
Warning: Hot surfaces inside. Contact may cause
burn. Do not touch.
Risk of Electric Shock. No user serviceable parts
inside. Refer servicing to qualified personnel.
Danger: Risk of explosion. DO NOT use in the
presence of flammable anesthetics
Use of This Documentation
This manual provides instruction for operation, maintenance, and troubleshooting procedures. Users
should be thoroughly trained in using this product and applicable medical procedures.

DOC # 25-3012 REV L 09/2021 Page 6of 29
Instruction manuals should be made available to the user(s) during the procedure. Follow all
instructions contained in this manual pertaining to the device, with particular
attention given to
the WARNINGS and PRECAUTIONS.
Warnings
Warnings are statements about conditions that could cause serious injury or death. The following warnings
apply:
Read this User’s Manual completely prior to use.
Do not use the aspirator in the presence of flammable anesthetics.
This device will not, in and of itself, produce significant weight reduction.
This device should be used with extreme caution in patients with chronic medical conditions, such as
diabetes; heart, lung, or circulatory system disease; or obesity.
The volume of blood loss and endogenous body fluid loss may adversely affect intra and/or postoperative
hemodynamic stability and patient safety. The capability of providing adequate, timely replacement is
essential for patient safety.
Precautions
This device is designed to contour the body by removing localized deposits of excess fat through small
incisions.
Use of this device is limited to those physicians who, by means of formal professional training or
sanctioned continuing medical education (including supervised operative experience), have attained
proficiency in suction lipoplasty.
Results of this procedure will vary depending upon patient age, surgical site, and experience of the
physician.
Results of this procedure may or may not be permanent.
The amount of fat removed should be limited to that necessary to achieve a desired cosmetic effect.
All reusable components of the device must be sterilized and all disposable components replaced before
using the device system on another patient.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
When performing a procedure, ensure that the collection canister(s) does not overfill. Aspirant or other
material entering the vacuum system may cause damage.

DOC # 25-3012 REV L 09/2021 Page 7of 29
Operate the B89025 VITRUVIAN™Ultimate Aspirator only with the supplied B89030 Hospital
Grade Power cord (or equivalent) “hospital grade” power cord.
1. Insert the universal end of the power cord into the receptacle on the back of the aspirator.
2. Connect the remaining end of the power cord to your AC outlet.
3. Black & Black Replacement Cord Part # B89030
Electrical connection should be made to a grounded outlet only.
Operate the B89025 VITRUVIAN™Ultimate Aspirator only at the specified voltage (110-120 or 220-
230 VAC). The aspirator’s operating voltage is user selectable and has been pre-set to the voltage
required at user site. Operating the B89025 at a voltage other than that for which it was
manufactured is dangerous and may damage or destroy the aspirator.
Never attempt to bypass or disable the B89025’s circuit breaker.
Do not restrict cooling fan.
Do not use the B89025 for a purpose other than that for which it was designed.
Do not attempt to service the B89025 unless advised Black & Black to do so.
Always use a biofilter(s) when performing aspiration. Replace biofilter(s) after each case. Optional
Black & Black Replacement Filter Part Number is B89104.
Contact Black & Black Surgical Customer Service at 770-414-4880 or 877-252-2517 if you have any
questions.
Operational Safety
The B89025 VITRUVIAN™Ultimate Aspirator needs special precautions regarding EMC and needs to
be installed and put into service according to the EMC information provided in this manual.
Portable and mobile RF communications equipment can affect the B89025 VITRUVIAN™Ultimate
Aspirator.
The use of accessories, transducers and cables other than those specified by Black & Black Surgical may
result in increased EMISSIONS or decreased IMMUNITY of the B89025 VITRUVIAN™Ultimate
Aspirator
The B89025 VITRUVIAN™Ultimate Aspirator should not be used adjacent to or stacked with other
equipment and that if adjacent or stacked use is necessary, the B89025 VITRUVIAN™Ultimate
Aspirator should be observed to verify normal operation in the configuration in which it will be used.
A thorough review of this entire instruction manual is essential prior to using this product.
Black & Black has taken great care to ensure the safety of the patient and staff. However, features
may not be
readily discernible without reviewing this documentation. Use of this equipment
should therefore not be
under taken until the user(s) is fully familiarized with the instructions for
assembly and operation. If you have any questions, contact Black & Black Surgical at (877)-252-

DOC # 25-3012 REV L 09/2021 Page 8of 29
Modifications
Modifications of any kind are NOT recommended and will void all warranties.
Damage or Loss in Shipment
Thoroughly inspect shipment immediately upon arrival. If goods are received short or in damaged
condition, it is important that you notify the transportation company and insist on a notation of the
loss or damage on the freight bill. Otherwise, it may be difficult to make a claim against the
Transportation
Company.
If concealed loss or damage is discovered, retain all packaging materials, notify the
transportation
company immediately, and request an inspection. The agent will make an inspection
and grant a
concealed damage notation. A concealed damage report must be made within seven (7)
days of shipment delivery. After seven (7) days, the transportation company reserves the right to
refuse any
claim for loss or damage.
Incorrect Items Shipped by Black & Black Surgical
Please check your shipment immediately for any shortage or incorrect items. If any discrepancies
exist,
notify Black & Black Surgical, (770) 414-4880, or info@blackandblacksurgical.com, at
once. Your prompt attention will ensure credit or exchange.
ALL DISCREPANCIES BETWEEN THE PACKING LIST AND THE PRODUCTS
RECEIVED MUST BE REPORTED WITHIN 48 HOURS OF RECEIPT OF THE
PACKAGE TO QUALIFY FOR A CREDIT.

DOC # 25-3012 REV L 09/2021 Page 9of 29
Policy on Returned Goods
NO RETURNS OR EXCHANGES WILL BE ACCEPTED UNLESS YOU HAVE RECEIVED
A RETURN OF MERCHANDISE AUTHORIZATION NUMBER (RMA) FROM BLACK &
BLACK. THIS NUMBER MUST BE ON THE RETURN SHIPPING LABEL AND ALL
WRITTENCOMMUNICATION.
Call Black & Black toll-free at 1-877-252-2517 to get a Return Merchandise Authorization (RMA)
your RMA.
ANY RETURN(S) WITHOUT RMA NUMBERS WILL NOT BE ACCEPTED AND WILL BE
RETURNED TO THE SENDER.
RETURN AUTHORIZATIONS ARE VALID FOR 30 DAYS.
Full credit will be issued for any item returned in 30 days that is in undamaged and saleable condition.
A restocking charge of 25% will be charged to any product returned between 31-90 days. A
credit
memo, based on the dollar amount, will be issued to the account.
OPENED PACKAGES OR BOXES containing disposable items are not returnable for credit or
exchange. Each package or sales unit specifically states “Not Returnable if Package Seal is
Broken.”
NO CREDIT WILL BE ISSUED ON ANY ITEM RETURNED AFTER 90DAYS.
Return Shipping
Return shipping, insurance, and handling is the responsibility of the customer. Credits will not be issued until
the product is received in acceptable condition. It is strongly recommended that the customer use a traceable
freight shipping method.
Please ship products in their original packaging, including documentation with all tags attached. Failure to do
so may result in a restocking fee to enable us to return the merchandise to a saleable condition.
Black & Black cannot be responsible for return shipping losses.
Each return must include the following information:
•Purchaser's name, telephone number and address
•B&B invoice number
•Invoice date
•RMA Number
•Purchaser purchase order number
•Quantity, catalog number and description of item
•Reason for return

DOC # 25-3012 REV L 09/2021 Page 10 of 29
Repair Program
If repairs are necessary due to damage other than that incurred during initial shipment (see
section
labeled DAMAGE OR LOSS IN SHIPMENT), contact Black & Black to return your unit. A
Return of Goods Authorization number must be obtained from Black & Black’s Customer Service
Department
prior to returning any merchandise. When requesting a Return Goods Authorization
number, please
follow the return policy as listed under the section labeled POLICY ON RETURNED
GOODS. Then,
please carefully repack and return it prepaid freight to:
Black & Black Surgical, Inc
Attn: repairs
RMA# _________
5175 South Royal Atlanta Dr.
Tucker, GA 30084
(770) 414-4880
(877) 252-2517
Repairs must be made by Black & Black Surgical or by an approved authorized agent. Attempting
repair without
prior authorization nullifies all warranties.
Warranty Policy
Black & Black products are manufactured for use only by qualified medical personnel who are
trained
in their use. This equipment carries a one-year warranty against defects from date of sale,
which warrants it to be free from defects in material and workmanship. This warranty is valid
only to the
original purchaser, and will be voided if transferred to a third party.
Any Black and Black product with such defects returned will be promptly repaired or replaced
at no charge to the customer. However, you are responsible for the cost of shipping the product to
us. The
warranty does not apply to damage caused by misuse, mishandling, improper operation,
excessive
voltage, and/or abuse of the product. Repairs or modifications performed other than by
Black & Black
or an approved authorized repair facility will nullify this warranty.
For details and warranty information, call Customer Service Department, (770) 414-4880 or
(877) 252-2517.

DOC # 25-3012 REV L 09/2021 Page 11 of 29
II. Operation
Intended Use
The VITRUVIAN™Ultimate Aspirator is intended to be used for: Aesthetic Body Contouring.
Intended User /Patient Population / Environment
Black & Black Surgical’s VITRUVIAN™Ultimate Aspirator should be handled and operated by
healthcare professionals completely familiar with use of the device. For prescription use only.
The intended patient population for this device includes any person seeking surgical, plastic, or liposuction
procedures for body contouring.
This device should be used in a hospital, clinical, surgical environment. NOT INTENDED FOR
HOME USE.
Contraindications
The devices are contraindicated for all the intended uses other than the ones claimed in these instructions for
use.

DOC # 25-3012 REV L 09/2021 Page 12 of 29
Unpacking
Figure 1
Your new VITRUVIAN™Ultimate Aspirator will come packaged similar to picture in Figure 1
shown above.
1. Lift the carton vertically as shown in Figure 1 to remove.
2. Carefully remove foam protection pieces from side of device.
3. Cut the flap on the rear side of the base carton as shown in Figure 2. Remove the foam from
around the base of the aspirator. Carefully move the aspirator off of the support foam and
roll out of box.
Please do not throw away / break down the rest of the packaging until device has been inspected
and tested.
Items Included with your device:
(1) Aspirator
(1) Power cord
(1) IV pole
(1) Foot pedal
(1) Accessories Basket
Figure 2
DOC # 25-3012 REV L 09/2021 Page 13 of 29
Initial Inspection, Set Up, and Testing
Your new aspirator was made to the highest standard and packaged to prevent shipping damage.
However, please perform the following before using.
Visually inspect the entire exterior for dents, scratches or any other damage.
Attach power connection on rear of device and connect cord
Insert the foot switch cord with tightening nut into the foot switch connections on either side
of the front of the device as seen on page 16.
Locate Vacuum control lever on front of machine and rotate clockwise all the way. This is
the highest level of vacuum.
Locate the power switch on front of device and switch to the “ON” position.
Locate the filter connection port on top of device and occlude the air flow.
At this point the numerical gauge on the front of the device should read:
oApproximately 29 inch (760 mm) of mercury at sea level. Subtract 1inch (25.4 mm)
for every 1000 feet (305 m) of altitude.
Once the above test is complete attach the dual canister basket to the mount on the side of
the device and place canister(s) into the basket.
Attach the filter to the top by screwing it on to the fitting.
oTighten filter until snug.
oCaution: Do not over tighten the filter. Over tightening can damage threads.
Attach canister connection tubing as shown on pages 18-19.
Occlude port P on canister 1 and repeat vacuum test. If all tubing is connected properly the
vacuum level will return to the same level as in the first test.
You are now ready to use the device.
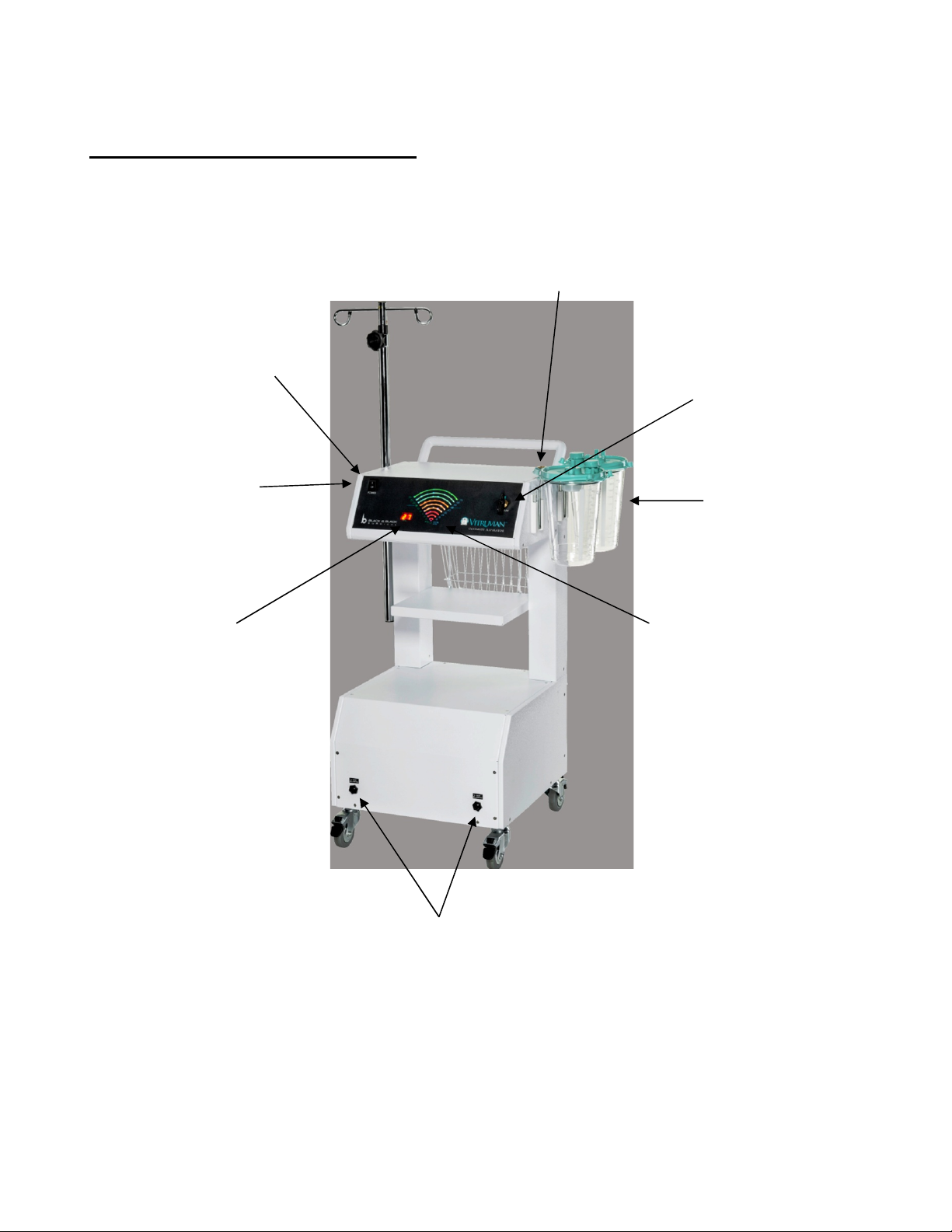
DOC # 25-3012 REV L 09/2021 Page 14 of 29
Operative Controls and Components
Front View
Power Switch
IV Pole Mount
Numeric Vacuum
Display in Inches
of Hg
Filter
Vacuum Control
Collection
Canisters
Graphic
Vacuum Display
Foot switch Connections

DOC # 25-3012 REV L 09/2021 Page 15 of 29
Front Panel
Power Switch
Numeric Vacuum
Display in Inches
of Hg
Vacuum Control
Graphic Vacuum
Display
(IN Hg / mm Hg.)

DOC # 25-3012 REV L 09/2021 Page 16 of 29
Foot switch Pictures
A
S
P
I
R
A
T
O
R
Foot Switch
FRONT
1. Feed foot pedal tubing through
the front of the tightening nut. Insert
tip of tubing into foot switch on
aspirator. Push it in so the tubing is
snug.
2. Push the tightening nut onto the
foot switch and tighten by turning
the nut clockwise.
3. Put 2nd tightening nut on unused
foot switch for backup
FRONT
BACK
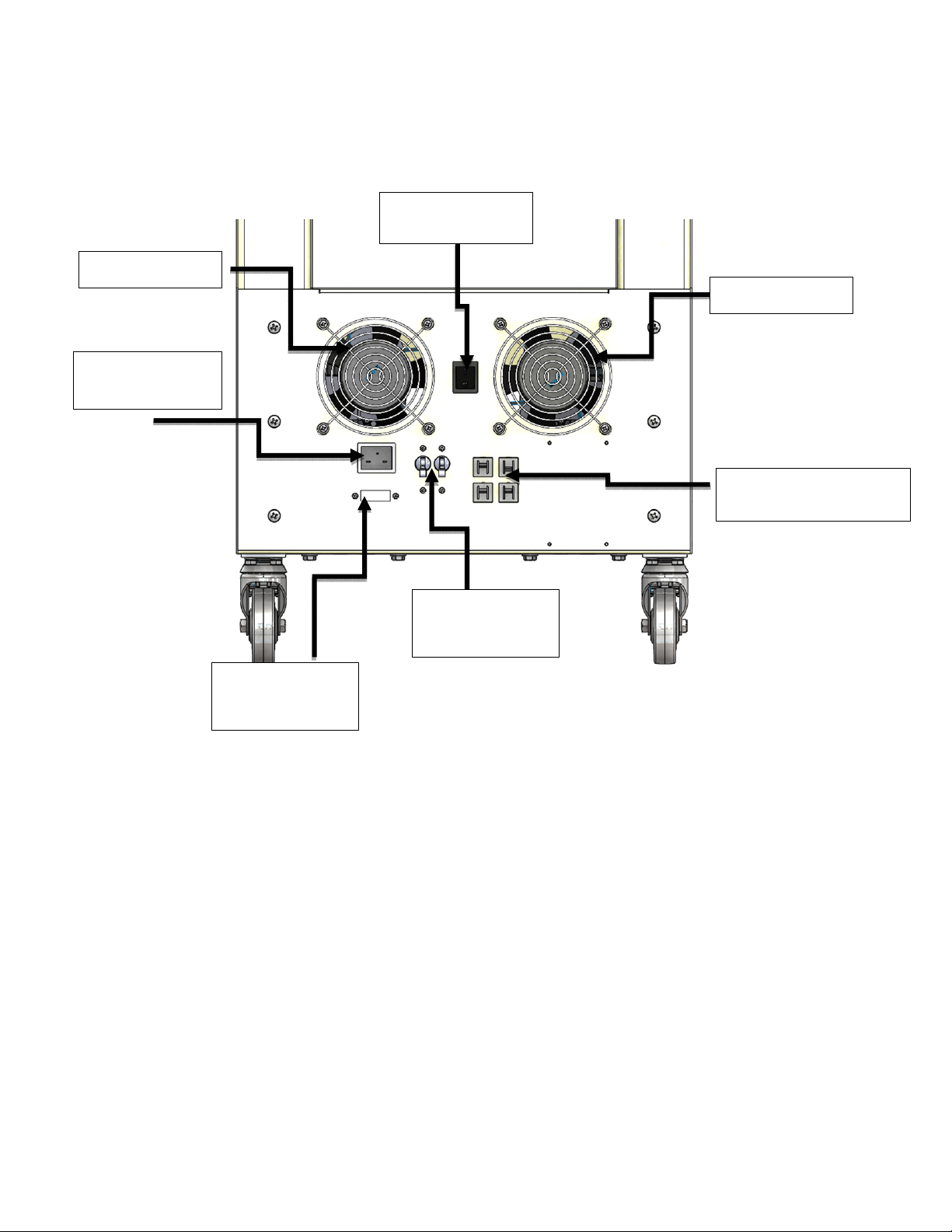
DOC # 25-3012 REV L 09/2021 Page 17 of 29
Rear View
Power cord
connection
Voltage
Selector
Cooling fan
Foot switch
control
Cooling fan
120V &230V circuit
breakers
Main Circuit
Breaker

DOC # 25-3012 REV L 09/2021 Page 18 of 29
For replacement canisters, filters or tubing contact Black & Black Surgical
Phone: 770-414-4880 Toll Free: 877-252-2517
Additional Features
1. A main breaker is installed which will cut power in the event of a severe power surge or
some other catastrophic event.
2. Each motor will work on either 120V or 230V.
3. Since each motor will operate on either 120V or 230V a separate circuit breaker for each
voltage and motor has been installed. In the event only one motor is affected by some event, the
remaining motor will continue to work. If the loss of one motor is noticed, immediately check for an
overflow situation. If there has been an overflow, change the canister, connection tubing and filter.
You should then be able to finish your case with the remaining motor.
Because of the features listed you should have years of safe and effective use, and hopefully should
never experience any of these circumstances.
Backup Operation of Device
Your device comes set up to use a foot pedal to control the power of the device
If for any reason the foot switch controls stop working, go to the rear of the unit and turn the
power switch labeled “FOOT SWITCH CONTROL” to the “OFF” position. Your device
will now be in aspiration mode until you turn the main power on the front of the device to
the “OFF” position.

DOC # 25-3012 REV L 09/2021 Page 19 of 29
(If using Black & Black parts) Canister Set-Up Illustration

DOC # 25-3012 REV L 09/2021 Page 20 of 29
Table of contents
Other BLACK & BLACK SURGICAL Medical Equipment manuals